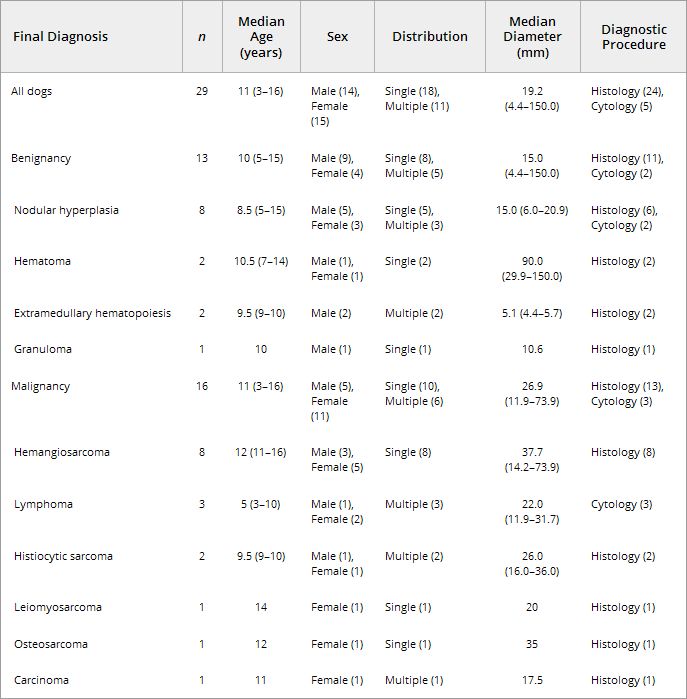
The vessel appearance was not significantly different between malignant and benign lesions. Similar pattern was found in 9 of the 16 malignant lesions (Fig 4B) and 10 of the 13 benign lesions
(Fig 1B). Different pattern was found in 4 malignant lesions. In the other 3 malignant and 3 benign lesions, vessel was invisible (Fig 2B). Among 4 dogs with different pattern, there were 3
hemangiosarcoma and 1 histiocytic sarcoma. All 3 hemangiosarcoma had aberrant wide or tortuous vessels in nodule (Fig 3B and C). One histiocytic sarcoma had tortuous vessel. Data for the vessel
appearance are given in Table 2.
Figure 4: Conventional ultrasound imaging (A) and perflubutane microbubbles-enhanced imagings (B–E) of splenic carcinoma. (B)
Immediately after injection, similar pattern vessel was visualized (arrow) in the lesion (arrowheads). (C) During the early vascular phase, the lesion was isoechoic (arrowheads) compared
with the surrounding normal parenchyma. (D) During the late vascular phase, the lesion became hypoechoic (arrowheads) compared with the surrounding normal parenchyma. (E) During the
parenchymal phase, the lesion was hypoechoic (arrowheads).
Figure 1: Conventional ultrasound imaging (A) and perflubutane microbubbles-enhanced imagings (B–E) of splenic nodular hyperplasia.
(B) Immediately after injection, similar pattern vessel was visualized (arrow) in the lesion (arrowheads). During both (C) the early vascular phase and (D) late vascular
phase, the lesion was isoechoic (arrowheads) compared with the surrounding normal parenchyma. (E) During the parenchymal phase, the lesion became hypoechoic (arrowheads).
Figure 2: Conventional ultrasound imaging (A) and perflubutane microbubbles-enhanced imagings (B–E) of splenic hematoma. (B)
Immediately after injection, no vessel was visualized in the lesion (arrowheads). During both (C) the early vascular and (D) the late vascular phase, the lesion was heteroechoic
(arrowheads). (E) During the parenchymal phase, the lesion became hypoechoic (arrowheads).
Figure 3: Conventional ultrasound imaging (A) and perflubutane microbubbles-enhanced imagings (B–E) of splenic hemangiosarcoma. During the
early vascular phase, (B) the tortuous vessel (arrow) and (C) aberrant wide vessel (arrow) were visualized, but the entire lesion was hypoechoic (arrowheads). (D) During the
late vascular phase, the lesion remained hypoechoic (arrowheads) with visualization of vessels (arrow). (E) During the parenchymal phase, the lesion became hypoechoic (arrowheads).
The enhancement patterns during the early and late vascular phases were significantly different between malignant and benign lesion (P= .02 and P < .001). There was no
significant difference during the parenchymal phase. Data for the contrast enhancement pattern after perflubutane microbubbles injection are given in Table 2.
In the early vascular phase, a hypoechoic pattern was found in 6 of the 16 malignant lesions and in none of the 13 benign lesions. All 6 hypoechoic lesions in the early phase were
hemangiosarcoma. Hypoechoic pattern was significantly associated with malignancy (P= .02) with sensitivity of 38% (95% CI, 25–38%) and specificity of 100% (95% CI, 84–100%). An isoechoic
pattern was found in 7 of the 16 malignant lesions and 10 of the 13 benign lesions. A heteroechoic pattern was found in 3 of the 16 malignant and 3 of the 13 benign lesions. Isoechoic and
heteroechoic patterns were not associated with malignancy or benignancy.
In the late vascular phase, a hypoechoic pattern was found in 13 of the 16 malignant lesions and 2 of the 13 benign lesions. Hypoechoic pattern in the late phase was significantly associated with
malignancy (P= .001) with sensitivity of 81% (95% CI, 66–90%) and specificity of 85% (95% CI, 65–95%). An isoechoic pattern was found in 1 of the 16 malignant lesions and 9 of the 13
benign lesions. Isoechoic pattern was significantly associated with benignancy (P= .001) with sensitivity of 69% (95% CI, 51–76%) and specificity of 94% (95% CI, 79–99%). A heteroechoic
pattern was detected in 2 of the 16 malignant lesions and 2 of the 13 benign lesions. Heteroechoic pattern was not associated with malignancy or benignancy.
In the parenchymal phase, a hypoechoic pattern was found in 15 of the 16 malignant lesions and 11 of the 13 benign lesions. A heteroechoic pattern was found in 1 malignant lesion and 1 benign
lesion. An isoechoic pattern was found in 1 benign lesion. There was no significant difference between malignant and benign lesions in the parenchymal phase.
Nodular hyperplasia (n= 8) was the most common benign lesion in this study (Fig 1). In the early vascular phase, 7 of the 8 nodules were isoechoic to the surrounding normal parenchyma
(Fig 1C). In 6 of the 8 dogs, nodules remained isoechoic in the late vascular phase (Fig 1D). In 2 of the 8 dogs, the nodules became hypoechoic in the late vascular phase. In the parenchymal
phase, all nodules were hypoechoic (Fig 1E). In 2 dogs, splenic nodules were diagnosed as hematoma (Fig 2). In the early and late vascular phases, both nodules were heteroechoic (Fig 2C and D).
In the parenchymal phase, both nodules became homogeneously hypoechoic (Fig 2E). In 2 dogs, splenic nodules were diagnosed as extramedurally hematopoiesis. Both nodules were isoechoic during the
early and late vascular phases. In the parenchymal phase, 1 was heteroechoic and another was isoechoic. In 1 dog, splenic nodule was diagnosed as granuloma. In the early vascular phase, the
nodule was isoechoic. It became hypoechoic during the late vascular and parenchymal phase.
Hemangiosarcoma (n= 8) was the most common malignant tumor in this study (Fig 3). In 6 of the 8 dogs, nodules were hypoechoic during the early and late vascular phases. In their nodules,
tortuous or aberrant wide vasculature was enhanced but the entire nodules were not enhanced (Fig 3B–D). In the other 2 dogs, nodules were heteroechoic in the early and late vascular phases. Seven
of the 8 nodules were homogeneously hypoechoic in the parenchymal phase (Fig 3E) and another was heteroechoic.
Seven of the 8 malignant nodules other than hemangiosarcoma were isoechoic in the early phase (Fig 4C). However, contrast enhancement in these 7 nodules rapidly decreased, and they became
hypoechoic in the late vascular phase (Fig 4D). Finally, all 8 lesions became hypoechoic in the parenchymal phase (Fig 4E).
Discussion
The results of our study suggest that evaluation of enhancement pattern in perflubutane microbubbles-enhanced ultrasonography has value in differentiating between malignant and benign splenic
nodules in dogs with high accuracy. However, the method for differentiating benign and malignant focal splenic lesions was quite different from that for the liver.13,14
The main result of this study is that there is no significant difference between benign and malignant lesions in the parenchymal phase. This finding is contrary to those of perflubutane
microbubbles-enhanced ultrasonography of the liver, in which hypoechogenicity in the parenchymal phase is suggestive of malignant tumors. Parenchymal enhancement in the liver after perflubutane
microbubbles injection is because of the distribution of the microbubbles in the Kupffer cells.9 The filling defect during the parenchymal phase created by the hepatic malignant tumor
is then because of a decrease in the number of Kupffer cells. Conversely, hepatic nodular hyperplasia, the most common benign focal liver lesion in dogs, shows contrast enhancement during the
parenchymal phase because the nodules contain Kupffer cells.13,14,16 On the other hand, splenic nodular hyperplasia, which is formed by hyperplastic lymphoid cells,17 became
hypoechoic during the parenchymal phase in our study. Although the precise mechanism of splenic parenchymal phase imaging is not fully determined, we speculate that the contrast defect during the
parenchymal phase created by splenic nodular hyperplasia might be because of a decrease of splenic macrophages. Therefore the parenchymal phase imaging was not useful for differentiation between
benign and malignant splenic lesions. In some cases, however, nodules that could not be visualized with conventional ultrasonography became clearly hypoechoic in the parenchymal phase. Therefore,
the parenchymal phase imaging could be useful for the detection of focal splenic lesions.
Another important finding is that the detection of hypoechoic nodules in the late vascular phase of perflubutane microbubbles-enhanced ultrasonography is suggestive of malignancy. In contrast,
detection of isoechoic nodules in the late vascular phase is suggestive of benign lesions. These findings agree with those of previous studies using the contrast agent sulphur hexafluoride
microbubbles.5,6 Sulphur hexafluoride microbubbles is a second-generation contrast agent that has been characterized to have splenic uptake in humans, allowing for parenchymal phase
imaging in the human spleen.18 In dogs, however, it was demonstrated that sulphur hexafluoride microbubbles allowed for vascular phase imaging but not for parenchymal phase
imaging.19 The vascular phase imaging with sulphur hexafluoride microbubbles could differentiate benign and malignant focal splenic lesions based on the finding that malignant tumors
were hypoechoic to the surrounding normal spleen parenchyma in the wash-out phase (30 seconds after injection of sulphur hexafluoride microbubbles).5,6 In the normal dogs and humans,
which have a sinusoidal spleen, a large sieve-like vascular reservoir is formed by an interconnected network of splenic sinusoids and red pulp spaces.17 It has been suggested that some
contrast agents are pooled in the splenic sinusoids for a time after intravenous injection.20 Therefore, we speculate that the lack of normal sinusoids combined with neoplastic
angiogenesis might be one of the causes of a malignant hypoechoic pattern during the late vascular phase.
The early vascular phase could also differentiate malignant and benign lesions with high specificity. Among them, hemangiosarcoma showed characteristic hypoechoic pattern during the early
vascular phase. This finding concurred with those for sulphur hexafluoride microbubbles-enhanced ultrasonography5,6 and contrast-enhanced computed tomography.21 This
hypoechoic area may correspond to the hemorrhagic or necrotic areas commonly associated with hemangiosarcoma. However, differentiation between hemangiosarcoma and hematoma should be done
cautiously. In a previous study with the contrast agent perflutren lipid microsphere, hemangiosarcoma and hematoma showed similar heteroechoic patterns during the peak enhancement.7 In
our study, likewise, 2 of the 8 hemangiosarcomas and both hematomas had similar heteroechoic patterns during the early vascular phase. Moreover, it was demonstrated that some cases of hematoma
exhibited a hypoechoic pattern with sulphur hexafluoride microbubbles.5,6 Although the exact reasons for these differences are uncertain, we speculate that they might be because of the
differences of contrast agents or patient populations in each study. Further studies are needed to clarify the criteria for discrimination between hemangiosarcoma and hematoma.
Evaluation of vessel appearance has no value in differentiating between malignant tumors and benign nodules. However, although some hemangiosarcomas had aberrant wide vessels, none of the other
malignant or benign lesions, including hematoma, had such vessels. This finding agreed with that of a previous study.5 It is quite difficult to evaluate the vessel pattern accurately
because of patient motion and the quite short duration of vascular enhancement in dogs. Vasculature pattern of focal splenic lesions should be evaluated cautiously but further studies are needed
to evaluate its diagnostic significance.
Footnotes
aDaiichi-Sankyo, Tokyo, Japan
bAplio XG, Toshiba Medical Systems, Tochigi, Japan
cPLT-704 AT, Toshiba Medical Systems
dPSK-375 BT, Toshiba Medical Systems
eStatMate, ATMS, Tokyo, Japan
Acknowledgment
This study was funded partly by Research Fellowships of the Japanese Society for the Promotion of Science.
References
-
1 Nyland TG, Mattoon JS, Herrgesell ER, Wisner ER. Spleen. In: NylandTG, MattoonJS, eds. Small Animal Diagnostic Ultrasound, 2nd ed. Philadelphia, PA: Saunders; 2002:128–143.
-
2 Blomley MJ, Albrecht T, Schlief
R, et al. Improved imaging of liver metastases with stimulated acoustic emission in the late phase of enhancement with the US contrast agent
SH U 508A: Early experience. Radiology 1999;210:409–416.
-
3 Hatanaka K, Kudo M, Minami
Y, Maekawa K. Sonazoid-enhanced ultrasonography for diagnosis of hepatic malignancies: Comparison with contrast-enhanced CT. Oncology 2008;75 (Suppl
1):42–47.
-
4 Von Herbay A, Barreiros AP, Ignee A, et al. Contrast-enhanced ultrasonography with SonoVue: Differentiation between benign
and malignant lesions of the spleen. J Ultrasound Med 2009;28:421–434.
-
5 Rossi F, Leone VF, Vignoli
M, et al. Use of contrast-enhanced ultrasound for characterization of focal splenic lesions. Vet Radiol
Ultrasound 2008;49:154–164.
-
6 Ohlerth S, Dennler M, Rüefli
E, et al. Contrast harmonic imaging characterization of canine splenic lesions. J Vet Intern Med
2008;22:1095–1102.
-
7 Ivancić M, Long F, Seiler
GS. Contrast harmonic ultrasonography of splenic masses and associated liver nodules in dogs. J Am Vet Med
Assoc 2009;234:88–94.
-
8 Yanagisawa K, Moriyasu F, Miyahara T, et al. Phagocytosis of ultrasound contrast agent microbubbles by Kupffer cells. Ultrasound Med Biol 2007;33:318–325.
-
9 Watanabe R, Matsumura M, Munemasa T, et al. Mechanism of hepatic parenchyma-specific contrast of microbubble-based contrast agent for
ultrasonography: Microscopic studies in rat liver. Invest Radiol 2007;42:643–651.
-
10 Watanabe R, Matsumura M, Chen
CJ, et al. Gray-scale liver enhancement with Sonazoid (NC100100), a novel ultrasound contrast agent; detection of hepatic tumors in a rabbit
model. Biol Pharm Bull 2003;26:1272–1277.
-
11 Hatanaka K, Kudo M, Minami
Y, et al. Differential diagnosis of hepatic tumors: Value of contrast-enhanced harmonic sonography using
the newly developed contrast agent, Sonazoid. Intervirology 2008;5:61–69.
-
12 Inoue T, Kudo M, Hatanaka
K, et al. Imaging of hepatocellular carcinoma: Qualitative and quantitative analysis of postvascular phase
contrast-enhanced ultrasonography with sonazoid. Comparison with superparamagnetic iron oxide magnetic resonance images. Oncology 2008;75 (Suppl 1):48–54.
-
13 Kanemoto H, Ohno K, Nakashima
K, et al. Characterization of canine focal liver lesions with contrast-enhanced ultrasound using a novel contrast agent-Sonazoid.
Vet Radiol Ultrasound 2009;50:188–194.
-
14 Nakamura K, Takagi S, Takiguchi M, et al. Contrast-enhanced ultrasonography for characterization of canine focal liver lesions. Vet Radiol Ultrasound 2010;51:79–85.
-
15 Nakamura K, Sasaki N, Takiguchi M, et al. Quantitative contrast-enhanced ultrasonography of canine spleen. Vet Radiol
Ultrasound 2009;50:104–108.
-
16 Fabry A, Benjamin SA, Angleton
GM. Nodular hyperplasia of the liver in the Beagle dog. Vet Pathol 1982;19:109–119.
-
17 Fry MM, McGavin MD. Bone
marrow, blood cells, and lymphatic system. In: McGavinMD, ZacharyJF, eds. Pathologic Basis of
Veterinary Disease, 4th ed. St Louis, MO: Mosby; 2007:743–832.
-
18 Lim AKP, Patel N, Eckersley
RJ, et al. Evidence for spleen-specific uptake of a microbubble contrast agent: A quantitative study in
healthy volunteers. Radiology 2004;231:785–788.
-
19 Ohlerth S, Ruefli E, Poirier
V, et al. Contrast harmonic imaging of the normal canine spleen. Vet Radiol Ultrasound 2007;48:451–456.
-
20 Görg C. The forgotten organ: Contrast enhanced sonography of the spleen. Eur J Radiol 2007;64:189–201.
-
21 Fife WD, Samii VF, Drost
WT, et al. Comparison between malignant and nonmalignant splenic masses in dogs using contrast-enhanced computed tomography.
Vet Radiol Ultrasound 2004;45:289–297.
Share this article / Teilen Sie diesen Artikel