Article added / Artikel hinzugefügt 01.10.2021
Generally Articles and Discussions about Osteosarcoma in Dogs
→ Evaluations of phylogenetic proximity in a group of 67 dogs with
osteosarcoma: a pilot study
Article added / Artikel hinzugefügt 01.10.2021
Generally Articles and Discussions about Osteosarcoma in Dogs
→ Canine Periosteal Osteosarcoma
Images added / Abbildungen hinzugefügt 02.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Pulmonary vessels
New subcategory added / Neue Unterkategorie hinzugefügt 02.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Pulmonary vessels
Images added / Abbildungen hinzugefügt 01.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Heart valvular diseases



Urine N-Telopeptide Excretion in Dogs with Appendicular Osteosarcoma

Lacoste, H., Fan, T. M., de Lorimier, L.-P. and Charney, S. C. (2006), Urine N-Telopeptide Excretion in Dogs with Appendicular Osteosarcoma. Journal of Veterinary
Internal Medicine, 20: 335–341.
doi: 10.1111/j.1939-1676.2006.tb02865.x

Canine appendicular osteosarcoma (OSA) is a commonly diagnosed cancer that is capable of inducing pathologic bone remodeling. Investigating surrogate indices of bone metabolism may contribute to
the diagnostic and therapeutic management of bone malignancies in companion animals. This study evaluated the excretion of N-terminal telopeptide (NTx), a marker of bone resorption that is
detected in urine. Sixty-three dogs with appendicular OSA were compared with 29 age-matched healthy dogs. Dogs with appendicular OSA had significantly higher baseline urine NTx excretion than
healthy controls ( P , .0001). In 17 dogs with OSA treated with either amputation or standardized palliative therapies, significant reductions in urine NTx excretion were observed, suggesting
that excessive bone resorption in dogs with OSA may be linked with focal skeletal osteolysis or its consequences. To identify any relationship between indicators of pathologic bone turnover,
baseline urine
NTx excretion was correlated with serum bone alkaline phosphatase (bALP) or radiographic tumor lengths at diagnosis. No significant correlations were identified between baseline urine NTx
excretion and either bALP or tumor length. The findings from this study suggest that high urinary NTx excretion may support the diagnosis of focal skeletal osteolysis in dogs, and reductions in
urine NTx excretion after treatment may reflect elimination or minimization of pathologic bone resorption
Appendicular osteosarcoma (OSA) is the mostcommon primary bone tumor of dogs, and leads to high patient morbidity and mortality from painful regional and distant metastatic disease, respectively. 1 Canine appendicular OSA typically involves the metaphyseal regions of long bones, and affected dogs commonly are presented for the evaluation of lameness attributed to bone pain. Although the precise mechanisms of malignant osteolytic pain have yet to be elucidated in companion animals, clinical evidence in human patients with cancer and murine tumor models supports the notion that tumor-induced bone resorption is linked with bone cancer pain.2–4
Canine OSA can cause severe skeletal lesions, which may be characterized radiographically as being osteolytic, osteoblastic, or mixed. Although useful for the identification of advanced skeletal pathology, plain-film radiographic studies can only reflect net cumulative skeletal activities.5,6 Because bone metabolism is a dynamic process, static radiographic images are neither sensitive nor accurate in characterizing the rate or magnitude of bone turnover. To better describe dynamic changes in bone metabolism, surrogate indices, which reflect real-time osteoblastic or osteoclastic activities, have been investigated for the diagnosis and treatment monitoring of pathologic skeletal processes in human patients. 5–9
Type I collagen is the primary structural protein of mineralized bone, accounting for approximately 90% of its organic matrix. During normal or pathologic bone turnover, osteoclasts enzymatically
degrade type I collagen, thereby releasing amino and carboxy terminal–derived fragments of type I collagen, designated as N-terminal telopeptide (NTx) and C-terminal telopeptide (CTx),
respectively. 10 These end products of bone resorption are excreted intact in the urine, and can be detected with commercial immunoassays previously validated for use in dogs.11 Although several
urine bone resorption markers have been identified, urine NTx is considered the most accurate marker of
bone resorption in humans with pathologic skeletal disorders.7–9 In human patients undergoing antiresorptive therapy for conditions such as osteoporosis or malignant osteolysis associated with
multifocal or diffuse skeletal metastases, evaluation of serial urine NTx concentrations can provide a sensitive and objective method to assess and monitor clinical response.10,12
Although urine NTx has proven useful for the initial and follow-up management of diffuse or multifocal malignant osteolytic diseases in human patients with cancer, its assessment and clinical
utility in cancerbearing animals has yet to be thoroughly described.13 In dogs with primary bone tumors such as OSA, identifying surrogate markers that reflect the degree of focal malignant
osteolysis may be clinically useful for early disease detection or therapeutic response assessment. The purpose of this study was to determine whether urine NTx excretion was different between
dogs with appendicular OSA and age-matched healthy control dogs. Additionally, to determine its specificity as a marker of malignant bone resorption, changes in urine NTx excretion were evaluated
in dogs with OSA
treated with either amputation or alternative palliative therapies. Finally, to determine whether any relationship
exists between urine NTx excretion and other indicators of pathologic bone turnover, baseline urine NTx excretion was compared to serum bone alkaline phosphatase (bALP) and plain-film
radiographic tumor length in dogs with OSA.
Materials and Methods
Baseline Urine NTx Excreti on Population
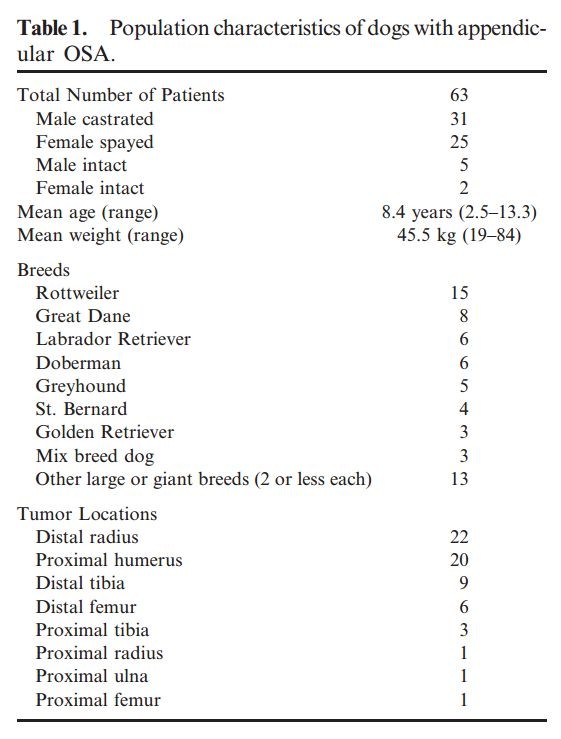
Sixty-three client-owned dogs were prospectively evaluated between January 2003 and December 2004. All dogs had a confirmed diagnosis of appendicular OSA by either histopathology (n 5 53) or
alkaline-phosphatase positive cytopathology (n 5 10). 14 None had previously received any form of definitive therapy defined as systemic chemotherapy, radiation therapy, or amputation.
All dogs with OSA (Table 1) were clinically staged with routine diagnostic tests including CBC, serum biochemistry, urinalysis, 3-view digital thoracic radiographs, abdominal ultrasonography, and
radiographs of the affected leg. Additional diagnostic tests (eg, urine culture and sensitivity testing) were performed as needed and
at the discretion of the clinician.
The control population used in this study consisted of 29 agematched healthy dogs with a median age and weight of 8.4 years (range, 2.5–13.3) and 31.6 kg (range, 17.2–57.0), respectively. These
control dogs belonged to house officers and clinicians, and on the basis of thorough histories, physical examinations, and routine blood work, all were considered in good health. None of the
control dogs at the time of urine collection, as well as for 6 months
after urine collection, had clinical evidence of pathologic skeletal disorders. Baseline urine NTx excretion levels were assessed from morning urine samples collected from all cancer-bearing dogs
and healthy controls.
For dogs diagnosed with OSA, all pet owners were informed of available treatment options, including standard-of-care amputation with adjuvant systemic chemotherapy, and traditional or
investigational palliative therapies. For patients not treated by amputation, pain management consisted of nonsteroidal antiinflammatory drugs (NSAIDs) with coarse-fraction radiation therapy,
opioids, aminobisphosphonates, or some combination of these treatments. For the purpose of this study, 2 specific therapeutic groups, totaling 9 dogs treated with amputation and 8 dogs
treated
with standardized palliative therapy, were included for posttreatment urine NTx analysis. The remaining 46 dogs either were part of other ongoing studies or did not have post-treatment urine
samples collected, and therefore, only baseline urine results were reported in this study. All dogs were handled and treated in accordance with the animal care guidelines of the University of
Illinois Institutional Animal Care and Use Committee.
Uri ne NTx Excretion in OSA Pop ulation Treated with Amputation
Of the 63 cancer-bearing dogs, 4 were treated with amputation alone and 5 dogs were treated with amputation and adjuvant systemic chemotherapy (doxorubicin, carboplatin, or a combination of
these). None of the 9 dogs treated with amputation had radiographic evidence of distant pulmonary metastatic disease at initial diagnosis. In addition to a baseline urine specimen collected 24
hours before amputation in all 9 dogs, a 2nd urine sample was collected a median of 19 days (range, 3–28) after amputation. Both urine samples were analyzed for NTx excretion.
NTx Excretion in OSA Population Treated with Standardized Palliative Therapy
Of the 63 cancer-bearing dogs, 8 dogs were prospectively treated with standardized palliative therapies consisting of coarse-fraction radiation therapy (800 cGy on day 1 and then every 7 days for
a total of 4 treatments),IV chemotherapy (doxorubicin hydrochloride,a 30 mg/m2 IV over 20 minutes on day 1 and then every 21 days for a total of 5 treatments), IV aminobisphosphonate (pamidronate
disodium,b 2 mg/kg IV on day 1 and then every 28 days for a total of 3 treatments), and an NSAID (deracoxib,c 1–2 mg/kg PO daily, beginning on day 1 of the protocol and continued indefinitely).
At initial diagnosis, none of the 8 dogs receiving standardized palliative therapy had radiographic evidence of distant pulmonary metastatic disease. A baseline urine specimen was collected
immediately before palliative therapy initiation in all 8 dogs, and a 2nd urine sample was collected 28 days later. Both urine samples were analyzed for NTx excretion.
Urine Cross- linke d N-telo peptide of Typ e I collagen (NTx ) Determinations
To minimize diurnal fluctuations in urine NTx excretion, urine samples from all patients were consistently collected in the morning, within a 4-hour window. Urine samples obtained either by
voiding, cystocentesis, or catheterization were centrifuged at 2000 3 g for 15 minutes at 4 uC. Urine supernatants then were separated and frozen in 2-mL polypropylene cryovials at 220 uC until
assayed. A commercially available immunoassayd validated in dogs was used to measure urine NTx excretion. 15 To normalize for fluctuations in renal clearance, urine NTx excretion was expressed in
relation to urinary creatinine concentration and reported in nanomolar bone collagen equivalents (BCE) per millimolar urine creatinine (nmol BCE/mM creatinine) as recommended by the manufacturer.
Serum Bone-Specific Alkaline Phosphatase Determination
Venous blood samples were collected by jugular venipuncture into preservative-free glass tubes and left to clot at room temperature for 15–20 minutes. Samples then were centrifuged at 2000 3 g
for 15 minutes at 4 uC, and serum was collected and stored in 2-mL polypropylene cryovials at 220 uC until assayed. Bonespecific alkaline phosphatase (bALP) activity was determined with a
commercial immunoassay kite previously validated for use in dogs.16 Serum bALP activities were reported in units per liter (U/L). Pathologic increases in bALP were established as $23 U/L,
as previously reported. 17–20
Tumor Length Determination
In 46 dogs with OSA, 1 investigator (HL) determined tumor lengths on the basis of plain-film radiographs. Pathologic bone changes identified on lateral radiographic views, including cortical and
medullary lysis, sclerosis, and periosteal proliferation, were used to define the most proximal and distal boundaries of malignant lesions. Tumor lengths were reported to the nearest 0.5 cm.
Statistical Analyses
Baseline urine NTx excretion from dogs with OSA (n 5 63) and healthy control dogs (n 5 29) were compared using the Wilcoxon rank sum test. In addition, the 2 study populations were compared for
differences in age and weight using the Wilcoxon rank sum test and for sex using the chi-square test. Differences in urine NTx excretion in dogs with OSA treated either with amputation or
standardized palliative therapies before and after therapy were compared using the signed rank test for paired data. All tests used for statistical significance were 2-sided. A Spearman rank test
was
used to evaluate the correlations between (1) urine NTx excretion and bALP and (2) urine NTx excretion and tumor length. Statistical analysis was performed using a commercial computer software.f
Statistical significance was defined as a P value , .05.
Results
Baseline Urine NTx Excretion Comparisons
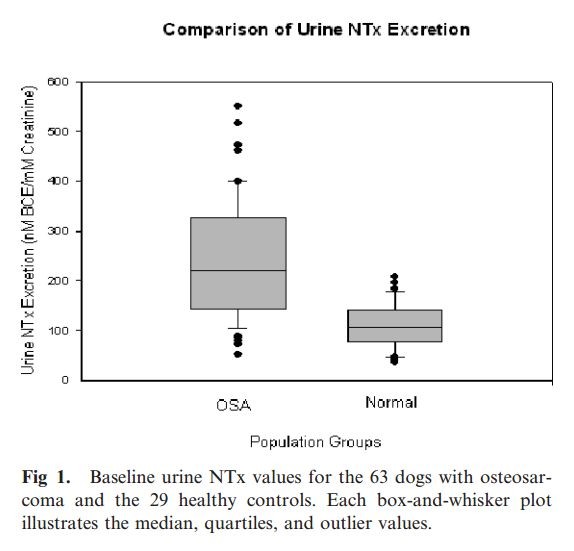
In dogs with OSA (n 5 63) the average baseline urine NTx excretion was 244.2 nM BCE/mM creatinine (range, 52.6–551.4). In healthy control dogs (n 5 29) the average urine NTx excretion was 109.3
nM BCE/ mM creatinine (range, 37.4–207.1). Dogs with appendicular OSA had significantly higher baseline urine NTx excretion levels than healthy control dogs (P , .0001) (Fig 1). Based on a
calculated 95% confidence interval using urine NTx excretion results from healthy control dogs, an upper normal limit for urine NTx excretion was determined to be 201.5 nM BCE/mM creatinine. Of
the 63 dogs with OSA, 37 (59%) had initial urine NTx excretion above the calculated normal upper limit for urine NTx excretion. No statistical difference was demonstrated between dogs with OSA
and normal dogs with regard to age (P 5 .4) or sex (P 5 .58). However, a significant difference in weight was identified between
these 2 populations, with OSA dogs weighing an average of 14.1 kg more than healthy controls (P 5 .0003).
Reductions in Urine NT x Excretion after Amputation
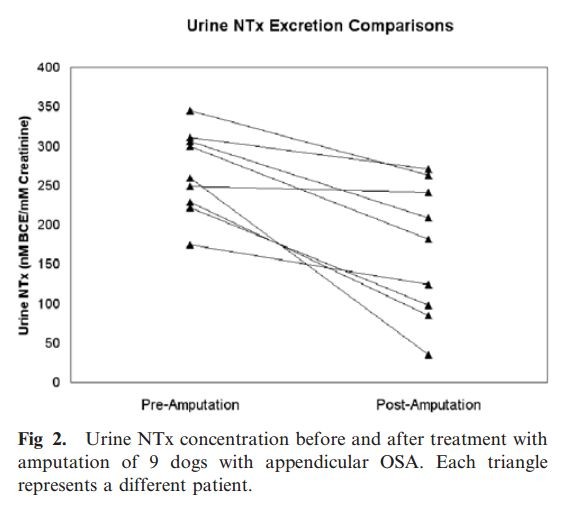
In 9 dogs with OSA treated by limb amputation, urine NTx excretion was assessed 24 hours before and at a median of 19 days (range, 3–28 days) after amputation. Average preamputation and
postamputation urine NTx excretion was 266.0 nM BCE/mM creatinine (range, 174.7–345.0) and 167.4 nM BCE/mM creatinine (range, 34.6–270.6), respectively. All 9 dogs experienced a reduction in
their urine NTx excretion after amputation, with an average percentage decrease of 38.3% (range, 3.0–86.6%), and the observed reductions were significant (P 5 .004) (Fig 2).
Reductions in Urine NTx Excretion after Standardized Palliative Therapy
Average prestandardized (day 0) and poststandardized (day 28) palliative therapy urine NTx excretions in 8 dogs were 335.0 nM BCE/mM creatinine (range, 110.0–551.4) and 109.9 nM BCE/mM creatinine
(range, 24.8–
291.2), respectively. All 8 dogs experienced a reduction in their urine NTx excretion after standardized palliative
therapy, with an average percentage decrease of 68.7% (range, 47.0–91.0%), and the observed reductions were
significant (P 5 .008) (Fig 3).

Correlation of Urine NTx Excretion with bALP and Tumor Length
In this study, 36 dogs with OSA had initial serum bALP activities averaging 28.3 U/L (range, 6.9–93.2). Based on prior studies in which the upper normal limit for serum bALP was defined as 23
U/L, 19 of the 36 dogs (53%) with OSA had increases in serum bALP activity.14–17 No significant correlation between baseline urine NTx excretion and baseline bALP activity could be identified in
these 36 dogs ( rs 5 .24, P . .05).
Similarly in this study, 46 dogs had initial radiographs of their appendicular tumor available for standardized analysis. The average tumor length was 7.9 cm (range, 1.5–13.5). No significant correlation between baseline urine NTx excretion and initial primary tumor length could be identified in these 46 dogs (rs 5 .26, P . .05).
Discussion
In the past decade, there has been growing interest in investigating the clinical utility of bone resorption
markers in human patients with cancer. The identification of sensitive surrogate markers for pathologic bone turnover has influenced the diagnostic and therapeutic management of focal and
multifocal malignant osteolytic disorders. Several studies have identified increased bone resorption markers in human patients with primary bone tumors.21–24 These studies underscore the
potential utility of such markers as noninvasive diagnostic screening tests for populations at risk. In addition to aiding diagnosis, bone resorption markers also may provide prognostic
information, allowing for accurate prediction of patient outcome.23,25
Although the incidence of primary bone tumors is far greater in dogs than people, the evaluation and clinical utility of bone resorptive markers in veterinary oncology remains limited. 13 Given
that OSA frequently is diagnosed and causes severe and extensive local osteolysis, it may represent an ideal tumor model for the clinical evaluation of bone resorption markers in cancer-bearing
dogs. In the present study, baseline urine NTx excretion was higher in most dogs with appendicular OSA than in age-matched healthy controls. To the authors’ knowledge, ours is the first study
that investigates the potential clinical relevance of bone resorption markers for assessment of focal malignant osteolytic disorders in dogs. Although dogs with OSA clearly had higher baseline
urine NTx excretion, there was overlap between normal healthy controls and dogs with malignant osteolysis. Of the 63 dogs with OSA, 26 (41%) had
urine NTx excretion ,201.5 nM BCE/mM creatinine, the calculated upper normal limit for urine NTx excretion. This finding demonstrates that although bone resorption is generally increased in dogs
with focal malignant osteolysis, baseline urine NTx excretion cannot be used as a sole discriminating diagnostic test for dogs with appendicular OSA. However, because the measurement of urine NTx
excretion is simple and noninvasive, it may be an attractive complementary diagnostic test in dogs with suspected or occult malignant osteolysis due to OSA and potentially other bone tumors.
Because urine NTx excretion reflects whole skeletal bone resorption, several potential reasons may account for the increased urine NTx excretion in dogs with OSA observed in this study.
Differences between OSA and healthy control dogs, and hence potential variables that might explain the discrepancies in urine NTx excretion, include the diagnosis of OSA, the presence of
osteolytic pain and its consequences, and body weight or skeletal mass. First, focal osteolysis directly caused by the growth and infiltration of OSA cells likely is a major contributor to the
observed higher urine NTx excretion in OSA dogs. However, this assumption is made on the basis of the mechanism of malignant osteolysis associated with diffuse or multifocal bone metastases in
humans whereby cancer cells directly or indirectly enhance osteoclastic activity and increase pathologic bone resorption.26 Whether a similar biological consequence occurs in primary bone
sarcomas remains to be determined in both humans and dogs. Second, a theoretical reason for increased urinary NTx excretion in dogs with OSA could be a consequence of chronic pain. Because dogs
often have reduced weight bearing on their affected leg due to pain, disuse osteopenia possibly could contribute to the increased urine NTx excretion observed in dogs with OSA. However, the
induction of disuse osteopenia in research studies requires dogs to be completely non–weight-bearing for several months.27 Although dogs with OSA in this study presented for lameness of variable
duration, none of the patients were completely non–weight-bearing, and disuse osteopenia was most likely not a common complication in this study population. Third, another hypothetical
consequence of chronic osteolytic pain could be the physiologic release of catecholamines and glucocorticoids,
promoting hormonally driven osteoclastic activity and skeletal bone resorption. 28 As demonstrated in osteoclastic cell cultures and rat models of restraint, excessive stimulation through either
b-adrenergic or glucocorticoid receptors can enhance osteoclastic activity.29–30 Finally, a sizeable difference in body weight or skeletal mass existed between the 2 study populations, with dogs
with OSA weighing on average 14.1 kg more than healthy controls. Because urine NTx excretion is a reflection of whole-skeletal bone resorption, dogs with greater skeletal mass may have higher
urine NTx excretion. Therefore, it remains a possibility that the weight discrepancy between the 63 tumor-bearing dogs and the 29 healthy controls may have biased the study results, and the
difference in urine NTx excretion could simply be a reflection of skeletal mass. However, a recent study failed to identify any significant differences in serum carboxy-terminal telopeptide of
type I collagen, another bone resorption marker, between Irish Wolfhounds and Pomeranians, suggesting that skeletal mass does not influence the rate or magnitude of bone
resorption. 31
Another potential factor that could account for a difference in the urinary NTx excretion of the 2 populations, but which was not critically evaluated in our study, is the presence of concurrent non-neoplastic bone remodeling processes. Common conditions of older dogs, such as periodontal disease and osteoarthritis, theoretically could account for a portion of urine NTx excretion. Because cancer-bearing and control dogs were age-matched, it is unlikely that these geriatric problems were significantly more common in the diseased dogs. However, the impact of such age-related conditions on urine NTx excretion currently is under investigation.
Regardless of the precise mechanism or mechanisms contributing to increased bone resorption, for a subset of cancer-bearing dogs in this study, some proportion of urine NTx excretion could be
directly attributed to the physical presence of the primary tumor or its associated processes. In 9 dogs treated by amputation, significant reductions in urine NTx excretion was achieved as early
as 3 days after surgery. The consistent association between primary tumor removal and reductions in urine NTx excretion underscores the participatory role of focal osteolytic lesions for
increased urinary NTx excretion in dogs with OSA. To further support the notion that malignant bone resorption contributes to urine NTx excretion, 8 dogs receiving palliative therapy also
experienced reductions in urine NTx excretion after 28 days of treatment. The decreases in urine NTx excretion and simultaneous alleviation of pain achieved in these dogs adds credence to the
hypothesis that uncontrolled malignant osteolysis contributes to urine
NTx excretion in dogs with OSA.
In this study, urine NTx excretion was compared to bALP and tumor length to identify possible correlations between these variables. Released from the plasma membrane of osteoblasts during
activation, bALP is associated with new bone formation. From prior studies, serum bALP is increased in some dogs with OSA and provides prognostic survival information.17,20 For 36 dogs with OSA
in which both urine NTx and serum bALP were compared, no significant correlation was identified. The failure to correlate urine NTx excretion and serum bALP in dogs with OSA is not unexpected,
because even in healthy dogs, these 2 bone turnover markers do not correlate with each other. 16 Dogs with OSA may experience even greater imbalances in bone turnover, with the resultant
uncoupling of bone resorption from bone formation. However, species differences may exist between urinary excretion markers of
bone resorption and serum markers of bone formation as demonstrated in a recent study in which urinary CTx
and serum bALP were well correlated in normal cats. 32
In addition to bALP, a relationship between urine NTx excretion and primary tumor length was investigated. For 46 dogs with OSA in which urine NTx excretion and primary tumor radiographs were
compared, no significant correlation was identified. This finding contrasts with what is reported in human patients with cancer who have skeletal metastases, in whom urine NTx excretion is
positively correlated not only with the number of skeletal lesions, but also with the extent of skeletal involvement. 7,33,34 For the dogs with OSA in this study, tumor length was based on gross
radiographic changes detected with plain-film radiography. Although appropriate for the evaluation of
advanced symptomatic lesions, plain-film radiography may lack the sensitivity to accurately delineate the extent
of more subtle lesions. Additionally, the typical lytic nature of many metastatic carcinomas of human patients with cancer differs markedly from the proliferative or mixed lesions common to
canine OSA, and it is conceivable that urine NTx excretion in dogs with OSA would be better correlated with the radiographic pattern associated with the primary tumor, as opposed to absolute
tumor length. In retrospect, perhaps more sensitive and objective imaging techniques such as computed tomography scans or magnetic resonance imaging for the assessment of tumor length,
quantitative uptake on bone scintigraphy, or dual energy X-ray absorptiometry (DEXA) scan for assessing bone mineral
density would have been more accurate to correlate with urine NTx excretion.35–39
Although this study provides new information regarding urine NTx excretion in dogs with malignant osteolysis, several limitations should be addressed. First, because urine NTx excretion
represents real-time wholeskeletal bone resorption, it is not specific for focal, malignant osteolytic lesions observed with canine appendicular OSA. Therefore, urine NTx excretion cannot be used
as an independent surrogate marker for appendicular OSA, but rather should be evaluated in conjunction with other noninvasive methods that assess bone health and metabolism. Second, urine
NTx
excretion can be influenced by many variables, including age, exercise level, systemic disease, and medical treatments. Although every attempt was made to minimize the differences between dogs
with OSA and age-matched healthy controls, inherent discrepancies between these 2 study populations existed and potentially may have biased the findings of this study.
Finally, the findings of this investigation support the notion that treating appendicular OSA with either amputation or standardized palliative therapies results in decreased urine NTx excretion.
However, because reductions in urine NTx in the majority of treated dogs with OSA resulted from the institution of combination therapies, it is not possible to determine the contribution of each
treatment modality for decreasing osteoclastic activity or viability. Both systemic chemotherapy and radiation therapy may exert inhibitory effects on osteoclast numbers and bone resorption.40,41
Furthermore, although reductions in urine NTx excretion after amputation or palliative therapies were significant, strong conclusions regarding the utility of urine NTx as an objective marker to
guide disease management cannot be made given the small number of dogs evaluated in this study. However using well-designed
prospective studies, surrogate indices reflecting malignant osteolysis may provide useful information to objectively monitor the therapeutic efficacy of palliative therapies in dogs with OSA.
Despite the higher incidence of primary bone tumors in companion animals, the clinical utility of bone resorption markers remains to be defined in veterinary oncology. Although the findings from this study are promising, additional prospective studies are required to validate the use of urine NTx excretion for managing dogs with OSA. Measurement of biochemical markers of bone metabolism, such as urine NTx, eventually may provide an easy, economical, and noninvasive tool for monitoring malignant skeletal diseases in companion animals.
Acknowledgments
We thank the staff of the Cancer Care Clinic for technical assistance and case management, and Dr. Anne Barger for critically reviewing the manuscript. This study was supported in part by the
American College of Veterinary Internal Medicine Foundation and the Illinois Governor’s Venture Technology Fund.
References
1. Chun R, de Lorimier LP. Update on the biology and
management of canine osteosarcoma. Vet Clin North Am Small
Anim Pract 2003;33:491–516.
2. Clohisy DR, Mantyh PW. Bone cancer pain. Cancer 2003;
97(3 Suppl):866–873.
3. Clohisy DR, Ogilvie CM, Ramnaraine ML. Tumor osteolysis
in osteopetrotic mice. J Orthop Res 1995;13:892–897.
4. Schwei MJ, Honore P, Rogers SD, et al. Neurochemical and
cellular reorganization of the spinal cord in a murine model of bone
cancer pain. J Neurosci 1999;19:10886–10897.
5. Demers LM. Bone markers in the management of patients
with skeletal metastases. Cancer 2003;97(3 Suppl):874–879.
6. Demers LM, Costa L, Lipton A. Biochemical markers and
skeletal metastases. Clin Orthop Relat Res 2003;415(Suppl):
S138–S147.
7. Demers LM, Costa L, Lipton A. Biochemical markers and
skeletal metastases. Cancer 2000;88(12 Suppl):2919–2926.
8. Lipton A, Demers L, Curley E, et al. Markers of bone
resorption in patients treated with pamidronate. Eur J Cancer
1998;34:2021–2026.
9. Costa L, Demers LM, Gouveia-Oliveira A, et al. Prospective
evaluation of the peptide-bound collagen type I
cross-links
Ntelopeptide and C-telopeptide in predicting bone metastases status.
J Clin Oncol 2002;20:850–856.
10. Hanson DA, Weis MAE, Bollen AM, et al. A specific
immunoassay for monitoring human bone resorption: Quantitation
of type I collagen cross-linked N-telopeptide in urine. J Bone Miner
Res 1992;7:1251–1258.
11. Allen MJ. Biochemical markers of bone metabolism in
animals: Uses and limitations. Vet Clin Pathol 2003;32:101–113.
12. Worsfold M, Powell DE, Jones TJW, et al. Assessment of
urinary bone markers for monitoring treatment of osteoporosis.
Clin Chem 2004;50:2263–2270.
13. Fan TM, de Lorimier LP, Charney SC, et al. Evaluation of
intravenous pamidronate administration in 33 cancer-bearing dogs
with primary or secondary bone involvement. J Vet Intern Med
2005;19:74–80.
14. Barger A, Graca R, Bailey K, et al. Use of alkaline
phosphatase staining to differentiate canine osteosarcoma from
other vimentin-positive tumors. Vet Pathol 2005;42:161–165.
15. Allen MJ, Allen LCV, Hoffmann WE, et al. Urinary
markers of type I collagen degradation in the dog. Res Vet Sci
2000;69:123–127.
16. Ladlow JF, Hoffmann WE, Breur GJ, et al. Biological
variability in serum and urinary indices of bone formation and
resorption in dogs. Calcif Tissue Int 2002;70:186–193.
17. Ehrhart N, Dernell WS, Hoffmann WE, et al. Prognostic
importance of alkaline phosphatase activity in serum from dogs
with appendicular osteosarcoma: 75 cases (1990–1996). J Am Vet
Med Assoc 1998;213:1002–1006.
18. Sanecki RK, Hoffmann WE, Hansen R, et al. Quantification
of
bone alkaline phosphatase in canine serum. Vet Clin Pathol
1993;22:17–23.
19. Sanecki RK, Hoffmann WE, Dorner JL, et al. Purification
and comparison of corticosteroid-induced and intestinal isoenzymes
of
alkaline phosphatase in dogs. Am J Vet Res 1990;
51:1964–1968.
20. Garzotto CK, Berg J, Hoffmann WE, et al. Prognostic
significance of serum alkaline phosphatase activity in canine
appendicular osteosarcoma. J Vet Intern Med 2000;14:587–592.
21. Wiklund T, Blomqvist C, Risteli L, et al. Type I and type III
collagen metabolites in adult osteosarcoma patients. Br J Cancer
1996;73:106–109.
22. Behrens P, Bruns J, Ullrich KP, et al. Pyridinoline crosslinks as
markers
for primary and secondary bone tumors.
Scand J Clin Lab Invest 2003;63:37–44.
23. Ferrari S, Zolezzi C, Pratelli L, et al. Urinary excretion of
pyridinium cross-links and serum osteocalcin levels in patients with
primary high-grade osteosarcoma. Calcif Tissue Int 2003;73:1–4.
24. Ac¸il Y, Springer I, Behrens P, et al. Differential enhancement of
collagen
crosslink excretion in cases of osteosarcoma and
chondrosarcoma. J Cancer Res Clin Oncol 2003;129:583–588.
25. Brown JE, Cook RJ, Major P, et al. Bone turnover markers
as predictors of skeletal complications in prostate cancer,
lung cancer, and other solid tumors. J Natl Cancer Inst 2005;
97:59–69.
26. Pandit-Taskar N, Batraki M, Divgi CR. Radiopharmaceutical
therapy
for palliation of bone pain from osseous metastases.
J Nucl Med 2004;45:1358–1365.
340 Lacoste et al
27. Grynpas MD, Kasra M, Renlund R, et al. The effect of
pamidronate in a new model of immobilization in the dog. Bone
1995;17(4 Suppl):225S–232S.
28. Sivagurunathan S, Muir MM, Brennan TC, et al. Influence
of glucocorticoids on human osteoclast generation and activity.
J Bone Miner Res 2005;20(3):390–398.
29. Takada T, Yoshinari N, Sugiishi S, et al. Effect of restraint
stress on the progression of experimental periodontitis in rats.
J Periodontol 2004;75:306–315.
30. Arai M, Nagasawa T, Koshihara Y, Yamamoto S, et al. Effect
of beta-adrenergic agonists on bone-resorbing activity in human
osteoclast-like cells. Biochim Biophys Acta 2003;1640:137–142.
31. Breur GJ, Allen MJ, Carlson SJ, et al. Markers of bone
metabolism in dog breeds of different size. Res Vet Sci 2004;
76:53–55.
32. DeLaurier A, Jackson B, Pfeiffer D, et al. A comparison of
methods for measuring serum and urinary markers of bone
metabolism in cats. Res Vet Sci 2004;77:29–39.
33. Costa L, Demers LM, Speicher T, et al. Biochemical
markers of bone turnover correlate with the extent of metastatic
bone disease. American Society of Clinical Oncology Annual
Meeting; May 1999.
34. Lipton A, Costa L, Ali SM, et al. Bone markers in the
management of metastatic bone disease. Cancer Treat Rev 2001;
27:181–185.
35. Davis GJ, Kapatkin AS, Craig LE, et al. Comparison of
radiography, computed tomography, and magnetic resonance
imaging for evaluation of appendicular osteosarcoma in dogs.
J Am Vet Med Assoc 2002;220:1171–1176.
36. Wallack ST, Wisner ER, Werner JA, et al. Accuracy of
magnetic resonance imaging for estimating intramedullary
osteosarcoma
extent in pre-operative planning of canine limb-salvage
procedures. Vet Radiol Ultrasound 2002;43:432–441.
37. Lauten SD, Cox NR, Brawner WR Jr, et al. Use of dual
energy x-ray absorptiometry for noninvasive body composition
measurements in clinically normal dogs. Am J Vet Res 2001;
62:1295–1301.
38. Berruti A, Dogliotti L, Osella G, et al. Evaluation by dual
energy X-ray absorptiometry of changed bone density in metastatic
bone sites as a consequence of systemic treatment. Oncol Rep
2000;7:777–781.
39. Bachrach LK. Dual energy X-ray absorptiometry (DEXA)
measurements of bone density and body composition: promise
and pitfalls. J Pediatr Endocrinol Metab 2000;13(Suppl 2):983–988.
40. Abramson EC, Chang J, Mayer M, et al. Effects of cisplatin
on parathyroid hormone- and human lung tumor-induced bone
resorption. J Bone Miner Res 1988;3:541–546.
41. Hoskin PJ, Stratford MR, Folkes LK, et al. Effect of local
radiotherapy for bone pain on urinary markers of osteoclast
activity. Lancet
2000;355:1428–1429.
Share this article / Teilen Sie diesen Artikel
Diese Webseite wurde mit Jimdo erstellt! Jetzt kostenlos registrieren auf https://de.jimdo.com