
Article added / Artikel hinzugefügt 01.10.2021
Generally Articles and Discussions about Osteosarcoma in Dogs
→ Evaluations of phylogenetic proximity in a group of 67 dogs with
osteosarcoma: a pilot study
Article added / Artikel hinzugefügt 01.10.2021
Generally Articles and Discussions about Osteosarcoma in Dogs
→ Canine Periosteal Osteosarcoma
Images added / Abbildungen hinzugefügt 02.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Pulmonary vessels
New subcategory added / Neue Unterkategorie hinzugefügt 02.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Pulmonary vessels
Images added / Abbildungen hinzugefügt 01.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Heart valvular diseases



The Use of Naturally Occurring Cancer in Domestic Animals for Research into Human Cancer: General Considerations and a Review of Canine Skeletal Osteosarcoma

Brodey, Robert S. “The Use of Naturally Occurring Cancer in Domestic Animals for Research into Human Cancer: General Considerations and a Review of Canine Skeletal Osteosarcoma.” The Yale Journal of Biology and Medicine 52.4 (1979): 345–361. Print.

Abstract
For many years, research into human cancer has concentrated on human patients and on artificially induced neoplasms in inbred murine hosts. Cancer, however, affects a great variety of mammals, particularly those that have been domesticated. Suchf naturally occurring neoplasms are common in dogs, cats, cattle, horses, etc., and offer fertile ground for studies relating to epidemiologyf, etiology, immunobiology, and therapy. Canine osteosarcoma is described in detail. The clinicopathologic features of this canine tumor closely approximate that of human osteosarcoma and thus make canine osteosarcoma an invaluable comparative model. Canine osteosarcoma and other naturally occurring tumors lie intermediate between the mouse models and human cancer. The use of these veterinary models in the future fabric of cancer research will broaden its base and will influence our conceptual approach to research and clinical options.
Neoplasia affects a broad spectrum of vertebrates from fish through amphibia, reptiles, birds, and mammals. It is not confined to only mouse and man. Each one of these naturally occurring
neoplasms offers yet another clue or clues in the complex biology of neoplasia-a group of diseases that for the most part still remain poorly understood, despite the massive application of
money and technology. The occurrence, therefore, of these neoplasms in a wide range of vertebrate hosts is of far more than passing interest. Cancer needs to be studied as a comparative
biological problem and not as a disease restricted to only a few species. The thrust of this paper will be twofold:
(1) to emphasize the great values inherent in using naturally occurring neoplasms in domestic mammals and
(2) to illustrate these values by reviewing canine osteosarcoma (OS) and comparing it to other experimental models and to its human counterpart. Veterinarians have treated cancer in their
animal patients for many decades. Only recently, however, has the great value of these animals been realized. These neoplasms occur naturally in largely outbred populations, a condition
closely analogous to that in man. Such tumors have been most closely studied in dogs, cats, and cattle but much is also known about neoplastic diseases in horses, sheep, goats, and
swine
[ 1]. For the purposes of this paper, however, I will concentrate on naturally occurring solid tumors in dogs.
Do these canine tumors have any value in helping to unravel the mysteries of cancer? The answer is a resounding yes. Aside from assisting in the treatment of a sick animal and helping a
distressed owner, the veterinarian may resolve specific questions relevant to human neoplasia. Let us first examine some experimentally induced OS in laboratory animals.
LABORATORY MODELS OF OS
This brief review does not attempt to include every experimental production of OS ever performed, but rather to familiarize the reader with some of the more important laboratory models.
1. Ridgway OS (ROS): Originally described in 1948 from a spontaneous inguinal mass in a male AK mouse, it has been established as a transplantable tumor and maintained in serial passage in
AKR mice. Therapeutic trials using ROS employ AKD2F hybrids as they withstand chemotherapy better than the inbred lines. Median lifespan of affected mice is 25-35 days. Up to 11 single drugs
are known that will cure mice of this neoplasm. ROS is a highly reproducible model to study drug interactions and tumor cell kinetics. Clinical staging of affected mice can simulate different
stages of human OS [2].
2. Moloney sarcoma virus inoculated into the tibial medulla of newborn Wistar Lewis rats results in OS in 97.7 percent of all animals. Lung metastasis develops in 77.9 percent after six
weeks. whereas after initial growth of the primary OS, the remaining 22.1 percent of the tumors regress. Cell-mediated immune response and serum blocking activity can be demonstrated in
vitro, and these results can be correlated with the clinical stage of the disease [3,4].
3. The implantation of methylcholanthrene into the embryonic odontogenic tissues of 30 Swiss mice, five months of age, produced a few OS although epithelial tumors were more common [5].
4. Cupric chelated N-hydroxy-2-acetylaminofluorene injected into the femoral medulla of 10-16 week old female Osborn-Mendel rats produced various types of sarcoma including OS in 21 of the 29
animals. Most neoplasms developed from the soft tissue adjacent to the bones where the carcinogen had leaked out from the trephine holes. Newer experiments are underway in which the trephine
sites are plugged immediately after injection of the carcinogen. A variety of other compounds of nickel, chromium, cobalt, and other standard carcinogens such as benzpyrene and
methylcholanthrene have also induced OS in mice [6].
5. X-irradiation, or the injection of various radionuclides have produced OS in rabbits, mice, dogs (Beagles), miniature swine, and macaques [7-11]. Young rabbits given Sr90 I.V. develop OS
of the external auditory meatus, a site where these boneseeking isotopes tend to accumulate because there is little bone turnover [8]. In miniature swine the daily feeding of mCi + Sr90
caused OS to develop in the bones of the skull. OS formation usually coincides with maximal irradiation necrosis of bone followed by perversion of the repair process [9].
In Beagles given Sr90, OS occurred at the three highest dose levels tested. The mean induction time was 266 days but the first histologic detection of OS may occur within 90 days. Only 4
percent of these tumors metastasized and the liver was the most common organ affected. Most OS arose from the endosteum with only a few being of metaphyseal origin. The major site of OS in
order of frequency was femur, lumbar spine, tibia, pelvis, sacrum, and thoracic spine. Whether or not irradiation activates a latent tumor virus is unknown [11].
6. FBJ-Moloney sarcoma virus is the only naturally occurring murine sarcoma virus. It is unique as it produces mostly OS. In 1966 FBJ virus was isolated from aCANINE OSTEOSARCOMA: A MODEL FOR
HUMAN OSTEOSARCOMA 347 spontaneous OS of the thoracic vertebra in a male CFI/Anl mouse. C-type viral particles similar to the murine oncorna viruses were detected electronmicroscopically.
Serial passage through newborn CF-l mice can shorten the latency period to 21 days after injection even though the average latent period is 40-60 days. In tissue cultures both focus-forming
sarcoma virus (FBJ-MSV) and associated non-focus forming leukemia virus (FBJ-MLV) are present. A delicate balance of FBJ-MSV and FBJ-MLV is needed for focus induction [12].
There is no question that such simplified laboratory systems have yielded a wealth of information to the cancer biologist. It is not the purpose of this paper to deny the great worth of such
experimental models. Rather, my purpose is to point out that there is a wide gap between these highly artificialized models and clinical human cancer. While further simplification of these
model systems allows for the construction of clear conclusions in a particular experiment, one must wonder whether some of these conclusions are applicable to the natural human disease, the
ultimate goal of such research. Indeed, such highly artificialized systems may at times be muddles rather than models. Thus while these mouse systems have a great deal to offer in cancer
research, they possess some serious limitations. Until very recently, research in OS, has been confined almost completely to the development of artificial models of OS in mice, rats, rabbits,
etc., whereas naturally occurring OS in dogs, cats, and other domestic species has been ignored. We must therefore, in addition, also seek other models that more closely approximate the
disease in man. Such animal models are commonplace and are seen almost daily by the average veterinary practitioner. By broadening the scope of cancer research into these available domestic
animal tumors, we can learn much more about etiology, epidemiology, immunology, metastasis, and therapy.
The advantages of these models can be outlined as follows: Neoplasia occurs naturally in these animals, most of which are outbred-thus closely simulating the human disease.
Many of these animals (dogs and cats) closely share the human environment. Because they live far shorter lives than man and tend to be restricted to their domiciles far more closely than
their owners, they offer excellent subjects for research into environmental causes of cancer. Indeed, a study in the Philadelphia area indicated that dogs with squamous cell carcinoma of the
tonsil were more closely associated with areas of air pollution than were dogs with primary lung and nasal carcinomas [13]. Furthermore, there is always the distinct possibility that
neoplasia in these animals may serve as an early warning system for a similar neoplasm in man. It is now very well known that the reproductive failures in many birds of prey was a clear
signal that the biosphere was heavily contaminated with DDT (dichlorodiphenyl trichloroethane) [14].
The biological behavior of many canine tumors very closely approximates that of their human counterparts. In the past decade there has been a great upsurge of interest in veterinary oncology.
Many veterinary schools have oncology units and good data retrieval systems. A national group, the Veterinary Cancer Society, was formed in 1973 to unite all those with an interest in these
diseases. The present-day therapy of many canine and feline neoplasms is multidisciplinary and involves all of the modalities used in the treatment of similar diseases in man. The literature
on these animal neoplasms has shown a tremendous increase and several texts on veterinary oncology are now in preparation. All of this means that the veterinary profession is now in an
excellent position to play an important role in many facets of cancer biology.
Furthermore, the World Health Organization has published a standardized system of histological diagnosis for canine neoplasms [15] and recently has completed work on TNM (primary tumor,
regional lymph node, distant metastases) clinical staging systems which are very similar to those being used in humans. There is increasing cooperation between members of the veterinary
profession both nationally and to some extent internationally.
Because of the much shorter life span of the dog, the manifestations of the neoplasm are compressed into a much shorter period. Likewise, evaluation of therapeutic results can be made in a
far shorter time period than in man. In general a one-year survival in the dog or cat is roughly equivalent to a 4-5 year survival in man.
Considerable epidemiological information on canine and feline neoplasms is already available, particularly that emanating from an extensive study in several California counties [ 16-18] as
well as from various university teaching hospitals [ 19]. It is well known that different breeds of dogs have different susceptibilities to various histologic types of tumors in a variety of
anatomic sites. For example, Boxer dogs have a broad range of susceptibility to a host of neoplasms (gastro-intestinal, lung, lymphoid, testicular, aortic body, thyroid, brain, cutaneous mast
cell tumors, osteosarcoma, etc.) [19,20]. Because of these observations and their apparently increased susceptibility to systemic mycoses [21-23], great interest has focused on possible
defects in cell-mediated immunity in this breed. Malignant melanoma of the oral cavity, a common canine tumor, has a predilection for heavily pigmented breeds, particularly the Cocker Spaniel
[19]. Suffice it to say that we have a wide range of naturally occurring tumors in our domestic animals. Up until now, the comparative value of these lesions has been largely wasted, and it
is now time to utilize some of these animals in comparative cancer research. The clinicopathologic features of canine OS have been described in considerable detail in the veterinary
literature [24-28]. I am reviewing the salient features of canine OS to familiarize physicians with the remarkable similarity between OS in man and dog.
INCIDENCE
OS appears to occur far more frequently in dogs than in man. Epidemiologic studies of Alameda County in the San Francisco region indicate that the annual incidence rate of bone tumors is
7.9/100,000 dogs, whereas the incidence of bone tumors in man is only 1. 1/ 100,000 [ 18]. At the University of Pennsylvania Veterinary Hospital (UPVH), OS accounted for 4.8 percent (123) of
2,550 histologically diagnosed canine neoplasms from 1952-1963 inclusive [19]. Furthermore, the vast majority of canine bone tumors are malignant, with OS being by far the most common
[27,28], whereas in man a large variety of benign bone neoplasms are also observed [29]. In Alameda County when basal and squamous cell tumors were excluded, the yearly cancer rates per
100,000 were 272.1 for humans compared to 360 for dogs [18].
Age
In one study of nearly 200 dogs, the median age was 7 years; average age 7.4 years; mode 9 years and the range 1-15 years. Ten percent of the dogs were less than 2 years of age and most of
these were between 18 and 24 months [27]. Osteosarcoma in dogsless than one year of age is rare. The life expectancy of many breeds varies considerably; in general, the giant breeds such as
St. Bernards and Great Danes havea lower life expectancy than the smaller breeds. The mean ages of the following breeds with OS were St. Bernard-6.2 years; Great Dane-6.5 years; Boxer-7.3
years; and Irish setter-8 years [27].
The best comparison of canine and human ages was formulated by Lebeau [30]. A 12-year-old human equals a 1-year-old dog, and a 24-year-old human equals a 2-yearold dog. From then on, each
rise of four years in man is equivalent to an increase of one year in the dog. Thus, a 56-year-old man would be equivalent to a 10-year-old dog. Using this comparison, it is clear that canine
OS occurs in a much older age group (mean human equivalent age of 44 years) than does human OS. In most large dogs, epiphyseal growth has ceased by 11 months, yet most OS occurs long after
that date. This later age of onset represents the major difference in the clinicopathologic features of OS between the two species, as most human OS occurs during childhood, adolescence, and
early adulthood [29].
In almost all canine non-osseous malignancies (excluding lymphomas), there is an increased risk with advancing age, but this is not so with OS where there is a sharp rise in risk from 5-9
years with a sudden decrease from then on, suggesting that a viral agent may be acting on dogs of large size and of certain genetic strains [19]

Breed
Using artificial selection, man has created hundreds of different canine breed types, all of which have different predilections for OS. That OS is a disease of large breeds has long been
recognized by practicing veterinarians. Indeed, the risk of OS in dogs greater than 80 lb has been estimated to be 61-185 times the risk for dogs weighing less than 20 lb [31]. In a review of 194
dogs with OS, only 4 percent weighed less than 25 lb [27]. Thus, OS is a sizerather than a breed-related disease. The major large breeds affected are St. Bernard, Great Dane, Boxer, German
Shepherd, and Irish setter. The proportions of these breeds affected vary with the population characteristics of dogs in the study area. The demonstration that OS was far more common in large
dogs was the basis of a study in children which indicated that children with osseous malignancies were of larger skeletal size than those with non-osseous neoplasms [32]. Achondroplastic dogs
such as Dachshunds, and Bassett Hounds have an exceedingly low incidence of OS [27]. The Beagle, an exceedingly common breed for laboratory research in cancer, has an exceedingly low natural
incidence of OS [33]. Mixed breed dogs also have a much lower than expected incidence of OS, probably because most of them tend to be small in size [27]. A study conducted at the University of
Pennsylvania Veterinary Hospital (UPVH) from 1952-1963 inclusive indicated a breed-specific crude rate per thousand for OS of 2.1 for all breeds compared to 6.9 for Boxers [19].
Genetic Factors
OS was found in six of 148 first-degree relatives of 21 index St. Bernard dogs with histologically proved OS at the University of Pennsylvania Veterinary Hospital, but was not found in any of the
110 first-degree relatives of 18 breed, age, and sexmatched controls [34]. Analysis of a composite pedigree constructed from individual four-generation pedigrees showed that all 21 index dogs
were related. The average coefficient of relationship for members of the OS-bearing group was significantly higher than that for members of the control group. The average coefficient of
inbreeding of the OS-bearing group was lower than, but not significantly lower than, that of the controls. This suggests that the presence of specific genes within certain family lines, and not
inbreeding per se, influences susceptibility to OS. The lack of information on entire litters precluded the formal testing of specific genetic hypotheses, but examination of the pedigree excluded
fully penetrant autosomal dominant and X-linked recessive inheritance. Other monogenic models or more complex modes, perhaps involving an interaction between genetic and environmental factors,
cannot be excluded, nor can vertical transmission of an infectious agent. Based on the familial pattern of occurrence of affected dogs, developing a strain with a high incidence of OS should be
possible by selective breeding. A method for identifying ancestors having a major contribution to the genome of the group of index dogs was also described.
Sex
In 272 dogs with OS at the University of Pennsylvania Veterinary Hospital, 160 were males, and 112 were females, a M:F ratio of 1.5:1.0 [35]. In Alameda County, California, canine bone tumors
comprised 2.85 percent of all cancers in males and 1.7 percent in females [17]. However, the sex ratios vary from breed to breed and these differences are presently being evaluated.
Site
In general, 77 percent of canine OS arises in the long bones, and 23 percent in the flat bones. The four major weight-bearing bones (radius, humerus, tibia, and femur) account for 70 percent of
the OS [27]. Generally the forelimb to hind limb ratio is nearly 2 to 1, but this ratio varies greatly from breed to breed, e.g., in 45 Boxers it was 1.2:1; whereas in 27 Great Danes it was
5.7:1. Furthermore, the long bone to flat bone ratio varies widely from breed to breed, e.g., Boxer 2.1:1 and Great Dane 26: 1. The five most common breeds appeared to fall into three major
groupings relative to site distribution: I. Irish Setters and German Shepherds; II. Boxers; III. Great Danes and St. Bernards [27]. Metaphyseal origin is far more common than diaphyseal. The
major sites in the forelimbs are quite specific, proximal humerus and distal radius; the elbow region is only infrequently affected. The predilection sites in the forelimb appear to correlate
with the approximate times of epiphyseal closure, e.g., the later the time of closure, the greater the frequency of OS. The closure times of the proximal and distal epiphyses of the humerus are
10-12 months and 8 months, whereas those of the proximal and distal radial epiphyses are 9 months and 10-12 months, respectively. In the hind limb, OS is commonly found on both the proximal and
distal femur and tibia.
The major sites of axial skeleton OS are the skull (24 of 42 in one study) and the ribs, particularly at the costochondral junction (8 of 42 cases). Other flat bones are also involved but with
far less frequency [27].
The tremendous variations in site frequencies is being studied further. Age, sex, breed, and site information has been collected from 1,400 dogs with OS [35]. Analysis of this data should give us
a great deal of epidemiological information on the interrelationships of these four parameters. The site predilections for naturally occurring OS in man and dog differs from those observed with
irradiation induction, suggesting a cause other than bone-seeking radionuclides [36].
Clinical Findings
The two major findings are lameness and swelling. The median duration of lameness is six weeks and 75 percent of the dogs have signs ranging from 1-9 weeks. Clinical signs have been observed for
six months, but this is rare. The lameness may or may not precede the swelling. The affected area contains a hard fusiform and often painless swelling. In severe cases lymphedema may be present
distal to the tumor. The growth of the mass is usually very rapid. Pathological fracture may be the presenting sign in a few very lytic tumors, or it may occur later in the clinical course.
Clinical signs of pulmonary involvement are very uncommon initially, although pulmonary signs are very common in the later stages [27]. Pulmonary metastases commonly lead to the development of
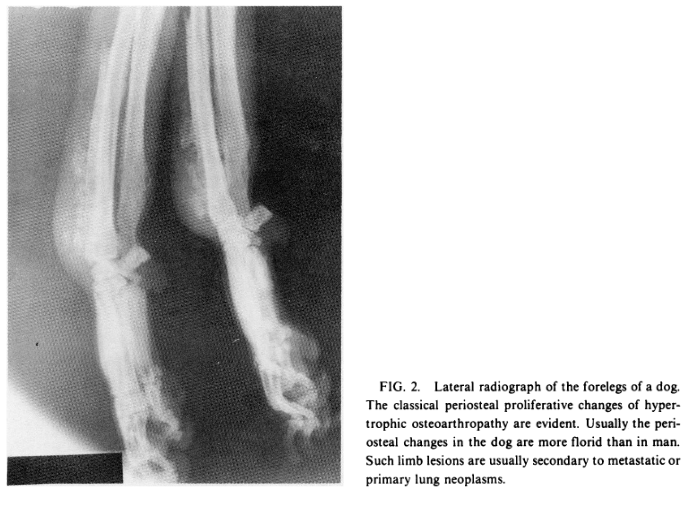
hypertrophic pulmonary osteoarthropathy (Fig. 2), a syndrome that has already been studied in man and dogs [27,37]. New concepts concerning its pathogenesis in man developed from studies of limb
blood flow initially performed in affected dogs [37].
Radiological Features
Canine OS shows all the features of a highly aggressive bone lesion with varying admixtures of osteolytic and osteoblastic changes. Purely lytic tumors account for approximately 5 percent of all
OS [38]; pathological fractures are most common in this group. Characteristic findings include sunbursting, Codman's triangle, irregular osteolysis, varying degrees of reactive periosteal new
bone formation, and origin at a metaphyseal site. All of these findings are not always present in any given case [27,28] (Figs. 3 and 4).
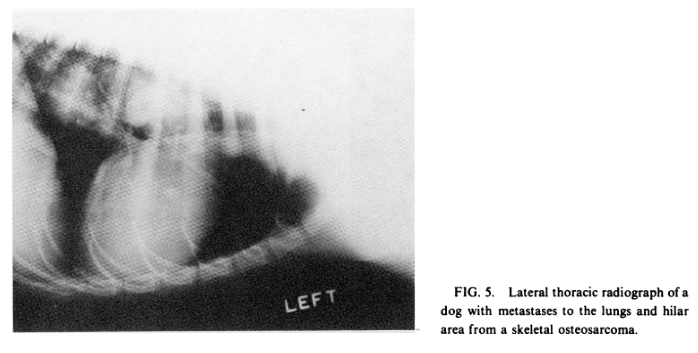
Pulmonary metastases (Fig. 5) are seen in less than 10 percent of dogs at the time of initial radiographic examination [25], but are extremely common at a median time of 18 weeks after limb
amputation [39].
Histopathologic Features
Canine OS is identical to its human counterpart. The characteristic finding is the direct formation of osteoid by the sarcoma cells (Fig. 6). Many canine OS also produce varying amounts of
chondroid and collagen, so that a mixed type of sarcoma is present [27,28].
CONTRIBUTING FACTORS
Medullary Infarcts These infarcts were first described in 1972 in four dogs, all of whom had superimposed sarcomas [40]. Since then, six more cases have been detected by the same authors. Nine of the 10 neoplasms were OS, and one was a fibrosarcoma. Most of the tumors arose in the tibia and femur. Most dogs were of the smaller breeds, and 4 of the 10 were Miniature Schnauzers, a breed rarely affected with OS. The cause of the infarcts is not yet known, but intramedullary occlusive vascular disease appears to play a role. Most dogs had multiple bony infarcts but only one site of sarcoma formation. One dog presented with two primary OS. Similar cases have also been reported in man [41]. We suspect that the stress of repair in the infarcted areas leads to a greatly increased risk of OS.


Metallic Bone Implants
Since the first canine OS associated with metallic bone implants was observed in 1959 [24], an additional 11 case reports have been published [42-44]. Corrosion products from these implants are
suspected to have a carcinogenic potential. A review of the case histories of the 12 reported canine cases revealed the following significant features. The latency periods between the
introduction of the metallic implant and the development of the OS ranged from 1-11 years with a median time of four years. Eight of the 12 tumors arose in the midshaft of a long bone, most often
the femur (in OS unassociated with metallic implants, the metaphysis is a far more common site than the diaphysis). The types of implants used were as follows: Jonas splint-5; Jonas splint plus
Steinman pin-1; bone plate-3; Steinman pin-2; and Steinman pin plus cerclage wire-1. The Jonas splint, a self-retaining intramedullary splint consists of three components. Metallurgical studies
have indicated that each component has a different composition and characteristics. These dissimilar metals in contact with each other caused an electrolytic reaction that accelerated the
corrosion of the more ionic metals and increased the dispersion of metallic products into the tissues. Chronic inflammation and drainage often developed after the insertion of this device.

In the past few years, six additional sarcomas (five osteosarcomas and one fibrosarcoma) have been observed in association with metallic implants at the UPVH [45]. Undoubtedly the number of
implant-associated OS actually observed in dogs greatly exceeds that which is recorded in the literature. The canine findings strongly suggest that sarcomas in man are also associated with such
devices far more often than the three case reports in the present literature [46-48] would indicate.
Trauma
Four or five OS arising at the site of a pre-existing healed fracture have been observed but such occurrences are exceedingly rare [26,35]. Immediate trauma seems to have no relationship
whatsoever to OS induction in dogs. Indeed, fractures are exceedingly common in dogs, primarily because of automobile injuries, whereas OS following such injuries (excluding metallic implant
fixation) is very rare. While a single traumatic incident plays no known role in the development of canine OS, small oft-repeated traumas associated with weight bearing may be of some importance.
The large and particularly giant breed dogs bear more weight on their forelimbs than on their hindlimbs, and correspondingly develop considerably more OS on their forelimbs. Weight bearing and
biomechanical balance are presently being studied by the author in 1,400 dogs with OS.
THERAPY
Surgical Therapy Surgical excision of OS in 65 dogs (limb amputation in 62 and rib removal in 3) resulted in a median survival of 18 weeks. When this was combined with a preoperative median
duration of clinical signs of six weeks, the total median duration of disease was 24 weeks. The survival times ranged from three to greater than 578 weeks. Only 13.8 percent lived more than 9
months after surgery, and 11 percent survived for one year. Two dogs were alive and free of disease more than two years postoperatively, and four other dogs died of causes unrelated to
osteosarcoma. No correlation could be found between postsurgical survival time and the duration of presurgical signs, the age, sex, or breed of the dog, the site of the tumor [39]; or the
radiographic features [38]. Histologic features of OS do not correlate with biological behavior except in a few OS where the tumor is predominantly fibroblastic [49].
Recently a few dogs have been treated by segmental resection of an affected ulna or fibula followed by chemotherapy [50].
Because many dogs with OS are euthanatized rather than dying a natural death, there is some variability in the survival results obtained, e.g., some owners may have their pets destroyed as soon
as radiographic evidence of lung metastasis is detected, whereas others may wait until terminal signs of disease become evident. For this reason, the first detection of metastatic disease
radiographically is a more objective and sensitive indicator of treatment failures. The median interval between amputation and the onset of radiographically visible metastatic lung disease was 73
days (range, 50-160 days) in nine dogs treated by amputation and doxorubicin [51].
Immunological Monitoring
Cell-mediated reactivity (CMR) and serum blocking activity (SBA) were assessed sequentially in 11 dogs treated surgically for OS. Six dogs received postoperative BCG and five served as controls.
SBA decreased significantly soon after surgery in both groups. No consistent relationship between CMR and SBA and clinical356 ROBERT S. BRODEY metastasis was detected in the postsurgical period
in either group [52,53]. Presently our sequential immunologic monitoring studies include lymphocyte transformation with various mitogens, leukocyte migration inhibition, and determinations of
effector T lymphocytes by the rosette technique both preoperatively and during adjuvant therapy. Immunological studies of canine OS are still in their infancy.
Immunotherapy
Very little work has been done in dogs with OS. Six dogs given freeze-dried BCG (Glaxo strain, 50-250 x 106 viable organisms) intravenously 1, 2, 4, and 8 weeks after amputation had a median
survival of 52 weeks (range 16-65 weeks) as compared to a mean survival of 11 weeks (range 6-17 weeks) for five control dogs [54].
Another group of six dogs was treated postsurgically with intradermal BCG (Trudeau strain, 1.5 x 107 viable organisms) at two-week intervals for one year. Their median survival was 40 weeks as
contrasted to five controls having a median survival of 13 weeks [53]. However, when the results of the two studies were combined, there was a statistically significant difference in survival
times (p<0.01) even though the strain of BCG and route of administration were different. More recently I.V. administration of Glaxo BCG given to 20 dogs following amputation for OS was
associated with a mean survival time of 25 weeks. Seven of these dogs were alive one year later [55]. Although these early results appeared encouraging, a recent controlled study of dogs with OS
indicated that survival times in 11 amputated dogs were similar to that of 12 dogs that were amputated and given postoperative BCG and autologous tumor homogenate [56].
Chemotherapy
The small numbers of dogs treated by chemotherapy that have been reported and the variation in treatment plans makes detailed assessment of the results rather difficult at present. In 1964, 17
dogs with malignancies of bone, most of which were OS, were treated with triethylene glycol diglycidyl ether. The drug was given intraarterially to five dogs, by regional perfusion to five dogs,
and using a technique to occlude the aorta and limit the drug to the cranial half of the body, it was administered to three others. Regression of the neoplasms occurred in 12 dogs but was
maintained for two months or more in only five of these. The longest period of regression was five months. Following perfusion there was local relief of pain, but edema often developed and in
four dogs gangrene was observed [57].
In 1978, 14 dogs treated by amputation and doxorubicin (30 mg/iM2 of body surface area) were reported. Doxorubicin therapy was initiated three weeks after surgery, and was given intravenously
every three weeks for 24 weeks. Drug therapy was discontinued if pulmonary metastases were detected radiographically. The median survival was 15 weeks (range 52-368 days) and 14 percent survived
more than one year [51]. These findings were almost identical to that following amputation alone in 65 dogs [39].
At present, several chemotherapy or chemotherapy-immunotherapy protocols are being evaluated, but the studies have yet to be completed.
A major problem in canine therapy trials involves the cost to the owner. Hopefully,
a greater appreciation of the value of these naturally occurring animal models will make money available to help owners defray some of the costs for multimodality therapy.
Radiation Therapy
Prophylactic lung irradiation using 600 R on two occasions at a one-week interval was found to be of no value in dogs with OS [58]. Seventeen dogs had their affected limbs treated with
fractionated doses of 4,000-4,500 R of X-irradiation from a 15 MeV linear accelerator giving 120-130 rads/minute with 100 percent penetration at 3 cm in tissue. Doses of 1,000 R were given at
weekly intervals. Reduction in pain was remarkable but mean pain-free survival was only four months with a range of 1-12 months [59].
Combined Modality Approach
Many exciting possibilities exist for future studies. If it can be shown that microand macrometastatic disease can be successfully treated medically, then trials concentrating on limb-saving
technics can begin. Combinations of irradiation with hyperthermia and radiation sensitizers may be of considerable value in treating the primary tumor with or without segmental resections.
Carefully designed multimodality clinical trials in dogs with OS should generate valuable biological data in a much shorter time than is possible in man.
PROGNOSTIC VARIABLES
A recent necropsy study of 144 untreated dogs with OS indicated that 49 had metastases, 44 of which involved the lungs [60]. Histologic subtype (excluding the fibrosarcomatous type) and grade,
mitotic index, amount of intercellular substance, numbers of peritumorous lymphoid and plasma cells, duration of clinical signs., breed, sex, and age of the dogs were not of prognostic
significance. The occurrence of pulmonary metastases was closely correlated with tumor diameter and volume as well as with extension of the OS into adjacent soft tissues and the origin of the OS
in bones of the hindlimbs. Future design of prospective randomized clinical trials in dogs should take these prognostic variables into account so that patient groups are biologically balanced to
ensure more accurate evaluation of the results.
ADVANTAGES OF STUDYING NATURALLY OCCURRING CANINE OS
1. OS is much more common in dogs than in man. It is a striking disease clinically and usually is easily recognized in the field. Centers carrying out therapeutic trials or other studies have not
had difficulty attracting cases from local veterinarians. At the UPVH we have seen approximately 50 dogs with OS during the past year alone. This great increase in case load has primarily been
due to the combined surgical, chemoand immunotherapy trial now under way.
2. There is a tremendous similarity in the clinical, radiological, and histological features of OS in dogs and man (Table 1), making an affected dog of tremendous value for a variety of
etiological, epidemiological, immunobiological, and therapeutic studies.
3. As both dog and man often share a common environment, similar causative factors may be operative in both species. Dogs with OS and other neoplasms may be important environmental monitors for
such diseases in man. Future epidemiological research should correlate human OS incidence data with that in other species in given geographic areas.
4. The large size of the canine patients makes them ideal for obtaining adequate samples of blood, tumor tissue, etc.-a problem that is often of major importance in laboratory rodents.
5. Veterinary oncology expertise has risen sharply in the last decade. An increasing number of veterinary schools and other institutions have oncology sections and many of these are carrying out
various therapy protocols. Since most of these protocols are on an outpatient basis, the research costs are greatly reduced.
6. Recent pedigree studies indicate we now have the necessary genetic knowledge to develop strains of dogs with heightened susceptibility to OS. Such strains would be invaluable, particularly in
studying etiological factors and host-tumor interactions in the early stages of the disease.

7. Many owners of dogs with OS would be very grateful to know that treatment or other related investigations in their pets might also help humans with OS. Because cancer will afflict one of every
four persons in the U.S., it is reassuring to such owners to know that therapeutic research in their pet may someday help them or their families.
8. Rat and mouse bone is non-osteonal, i.e. lacks Haversian canals, unlike bone in rabbits, all domestic mammals, and man [61]. Thus the development of OS in species having osteonal bone most
closely approximates OS in man.
DISADVANTAGES
1. The large size of affected dogs (95 percent weigh more than 30 lb) means that housing them will often demand larger kennel areas and increased feeding costs. Similarly, drug costs will be
greater because of the large size of many of the patients when compared to laboratory rodents. However, most of these objections can be circumvented by relying primarily on outpatient
studies.
2. Beautifully controlled studies such as those in experimental murine hosts are not as likely in canine OS because of the variability of owner responses to the stress of treatment. Similar
problems are encountered with human patients and their families.
OTHER NATURALLY OCCURRING OS
Dogs and humans are the two mammalian species most commonly affected. Domestic cats are the third most common species to develop OS. The most common sites in cats appear to be the humerus, femur,
tibia, and skull [62]. Flat bones are involved as commonly as long bones, and the hindlimbs are involved more often than the forelimbs [35]. It has been suggested that feline OS has a lower
metastatic potential than in man or dog [51], but more cases must be studied before this can be documented. Many of the clinical, radiological, and pathological features of feline OS closely
approximate those of OS in man and dog. The major difference is that OS in cats occurs primarily in older animals [62]. Almost no information relevant to therapy has been published. Three cats
with limb OS were treated by amputation and postoperative doxorubicin. One was euthanatized 180 days after surgery because of unrelated disease, another at 143 days because of lung metastases. A
third cat was alive 436 days postoperatively despite the presence of lung metastasis [51].
Osteosarcoma developed in the thoracic vertebrae of three piglets and in the lumbar vertebrae of another piglet in England. Ages of affected animals were 4, 5, 9, and 11 weeks. Two of the piglets
originated from the same farm. In India OS of the ribs was diagnosed in two pigs that were nine months old [63]. More careful studies of young pigs may reveal that OS is more common than is
presently realized. Interestingly the age incidence of pigs with OS closely approximates that of humans with OS.
In sheep OS is rare, although chondrosarcoma arising from the rib cage has been reported in a number of instances [64]. The ultrastructure of an OS obtained from the pelvic region of an adult ewe
was studied. The pattern of distribution of the organized interchromatin-like granules in almost every tumor cell nucleus was similar to that observed in human rhabdomyosarcoma where a viral
origin is suspected [65].
In all other domestic mammals OS is exceedingly rare; however, OS has been reported in a few other subhuman primates, e.g., an adult male squirrel monkey had an OS of the proximal humerus. It was
reported to be clinically well 20 months after amputation. A 10-month-old lemur developed an OS in the carpal region. No metastases were detected at post mortem [66].
CONCLUSION
This review of naturally occurring canine OS (it also occurs in cats, swine, etc.) and artificially induced OS in rabbits, rodents, swine, and dogs indicates that a wealth of biological data
already exists on this deadly disease. It is the responsibility of all of us interested in the biology and treatment of cancer to glean as much knowledge as possible from these mammalian systems,
each of which offers many clues. Such a broad-based knowledge will undoubtedly influence our conceptual approach to research, and clinical options. To concentrate solely on OS in humans and
rodents greatly limits these options, for as Robert Burns so clearly stated, "the best laid schemes of mice and men gang aft a-gley."
REFERENCES
1. Moulton JE: Tumors in Domestic Animals. Berkeley, Los Angeles, London, Univ of Calif Press, 1978, pp 1-465
2. Laster WR: Ridgway osteogenic sarcoma-a promising laboratory model for special therapeutic trials against an advanced-staged, drug-sensitive animal tumor system. Cancer Chemother Res
5:151-168, 1975
3. Friedlaender GE, Mitchell MS: A laboratory model for the study of the immunobiology of osteosarcoma. Cancer 36:1631-1639, 1975
4. Friedlaender GE, Mitchell MS: A virally induced osteosarcoma in rats: A model for immunological studies of human osteosarcoma. J Bone & Joint Surg 58:295-302, 1976
5. Levy BM. Mohammed Cl, Broome AO, et al: Experimental osteosarcoma in mandibles of mice. Oral Surg, Oral Med & Oral Path 19:623-627, 1965
6. Stanton MF: Primary tumors of bone and lung in rats following local deposition of cupric-chelated N-hydroxy-2acetylaminofluorene. Cancer Res 27:1000-1006, 1967
7. Mole RH: Bone tumour production in mice by strontium-90: Further experimental support for a two-event hypothesis. Brit J Cancer 17:524-531, 1963
8. Kshirsagar S, Vaughan J, Williamson M: The occurrence of squamous carcinoma and osteosarcoma in young rabbits injected with 90 Sr (50-100uc/kg). Brit J Cancer 19:777-786, 1965
9. Howard EB, Clark WJ, Karagianes MT, et al: Strontium-90-induced bone tumors in miniature swine. Rad Res 39:594-607, 1969
10. Thurman GB, Mays CW, Taylor GN, et al: Growth dynamics of beagle osteosarcomas. Growth 35:119-125, 1971
11. Pool RR, Williams RJR, Goldman M: Induction of tumors involving bone in beagles fed toxic levels of strontium90. Am J Roentgen, Radium Ther & Nucl Med 118:900-908, 1973
12. Levy JA, Hartley JW, Rowe WP, et al: Studies of FBJ osteosarcoma virus in tissue culture. 1. Biologic characteristics of the "C"-type viruses. JNCI 51:525-539, 1973
13. Reif JS, Cohen D: The environmental distribution of canine respiratory tract neoplasms. Arch Environ Health 22:136-140, 1971
14. Grossman ML Hamlet J: Birds of Prey of the World. New York, Bonanza Books, 1964, pp 183, 186
15. World Health Organization: International Histologic Classification of Tumors of Domestic Animals 50:1-142, 1974
16. Dorn CR, Taylor DON, Frye FL, Hibbard HH: Survey of animal neoplasms in Alameda and Contra Costa counties, California. 1. Methodology and description of cases. JNCI 40:295-305, 1968
17. Dorn CR, Taylor DON, Schneider R, et al: Survey of animal neoplasms in Alameda and Contra Costa counties, California. 11. Cancer morbidity in dogs and cats from Alameda county. JNCI
40:307-318, 1968
18. Dorn CR: Epidemiology of canine and feline tumors. JAAHA 12:307-312, 1976
19. Cohen D, Reif JS, Brodey RS, et al: Epidemiological analysis of the most prevalent sites and types of canine neoplasia observed in a veterinary hospital. Cancer Res 34:2859-2868, 1974
20. Howard EB, Neilsen SW: Neoplasia of the boxer dog. Am J Vet Res 26:1121-1131, 1965
21. Robinson VB, McVicker DL, Peterson JC: Some aspects of the epizootiology of histoplasmosis in two boxer breeding kennels. JAVMA 119:196-200, 1951
22. Maddy JT: Disseminated coccidioidomycosis of the dog. JAVMA 131:483-489, 1958
23. Swerczek TW, Schiefer B, Neilsen SW: Canine actinomycosis. JAVMA 115:955-970, 1968
24. Brodey RS, McGrath JT, Reynolds H: A clinical and radiological study of canine bone neoplasms. Part 1. JAVMA 134:53-71, 1959
25. Owen LN, Stevenson DE: Observations on canine osteosarcomata. Res Vet Sci 2:117-129, 1961 26. Brodey RS, Sauer RM, Medway W: Canine bone neoplasms. JAVMA 143:471-495, 1963
27. Brodey RS, Riser WH: Canine osteosarcoma, a clinicopathologic study of 194 cases. Clin Orthop 62:54-64, 1969
28. Ling GV, Morgan JP, Pool RR: Primary bone tumors in the dog: A combined clinical, radiographic and histologic approach to early diagnosis. JAVMA 165:55-67, 1974
29. Dahlin DC: Bone Tumors. 2nd ed. Springfield, IL, CC Thomas, 1967, pp 1-285
30. Lebeau A: L'age du chien et celui de l'homme, essai de statistique sur la mortalite canine. Bull Acad Vet France 26:229-232, 1953
31. Tjalma RA: Canine bone sarcoma: Estimation of relative risk as a function of body size. JNCI 36:1137-1150, 1966
32. Fraumeni J: Stature and malignant tumors of bone in childhood and adolescence. Cancer 20:967-973, 1967
33. Mays CW, Taylor GN: Low natural incidence of osteosarcomas in beagles. Res Radiobiol COO: 1 19-231, 1964
34. Bech-Nielsen S, Haskins ME, Reif JS, et al: Frequency of osteosarcoma among first-degree relatives of St. Bernard dogs. JNCI 60:349-353, 1978
35. Brodey RS: University of Pennsylvania School of Veterinary Medicine. Unpublished data, 1979
36. Thurman GB, Mays CW, Taylor GN, et al: Skeletal location of radiation-induced and naturally occurring osteosarcomas in man and dog. Cancer Res 33:1604-1607, 1973
37. Holling HE, Brodey RS, Boland HC: Pulmonary hypertrophic osteoarthropathy. Lancet (Dec 9): 1269-1274, 1961
38. Biery DN, Rhodes WR, Brodey RS: University of Pennsylvania School of Veterinary Medicine. Unpublished data, 1978
39. Brodey RS, Abt DA: Results of surgical treatment in 65 dogs with osteosarcoma. JAVMA 168:1032-1035, 1976
40. Riser WH, Brodey RS, Biery DN: Bone infarctions associated with malignant bone tumors in dogs. JAVMA 160:411-421, 1972
41. Gall SJ, Weintraub HP, Proppe KH: Malignant fibrous histiocytoma and pleomorphic sarcoma in association with medullary bone infarcts. Cancer 41:607-619, 1978
42. Banks WC, Morris E, Herron MR, et al: Osteogenic sarcoma associated with internal fracture fixation in two dogs. JAVMA 167:166-167, 1975
43. Harrison JW, McLain DL, Hohn RB, et al: Osteosarcoma associated with metallic implants, report of two cases in dogs. Clin Orthop 116:253-257, 1976
44. Sinibaldi K, Rosen H, Liu SK, et al: Tumors associated with metallic implants in animals. Clin Orthop 118:257-266, 1976
45. McNeil BJ: University of Pennsylvania School of Veterinary Medicine. Personal communication, 1978
46. McDougal A: Malignant tumor at site of bone plating. J Bone & Joint Surg 38B:709-713, 1956 47. Delgado ER: Sarcoma following a surgically treated fractured tibia: A case report. Clin
Orthop 12:315-318, 1958
48. Dube VE, Fisher DE: Hemangioendothelioma of the leg following metallic fixation of the tibia. Cancer 30:1260-1266, 1972
49. Misdorp W: Netherlands Cancer Institute, Amsterdam. Personal communication, 1978
50. Theilen GH: University of California School of Veterinary Medicine, Davis, California. Personal communication, 1978
51. Madewell BR, Leighton RL, Theilen GH: Amputation and Doxorubicin for treatment of canine and feline osteogenic sarcoma. Europ J Cancer 14:287-293, 1978
52. Fidler IJ, Brodey RS, Bech-Nielsen S: In vitro immune stimulation-inhibition to spontaneous canine tumors of various histologic types. J 1mm 112:1051-1060, 1974
53. Bech-Nielsen S, Brodey RS, Fidler IJ, et al: The effect of BCG on in vitro immune reactivity and clinical course in dogs treated surgically for osteosarcoma. Europ J Cancer 13:33-41,
1977
54. Owen LN, Bostock DE: Effects of intravenous BCG in normal dogs and in dogs with spontaneous osteosarcoma. Europ J Cancer 10:775-780, 1974
55. Owen LN, Bostock DE, Lavelle RB: Studies on therapy of osteosarcoma in dogs using BCG vaccine. J Am Vet Radiol Soc 18:27-29, 1977
56. Weiden PL, Storb R, et al: Canine osteosarcoma. Results of amputation with and without adjuvant immunotherapy. Cancer Immunol, Immunother 5:181-186, 1978
57. Owen LN: The treatment of malignant tumors of bone in the dog by intra-arterial injection of perfusion of epodyl (triethyleneglycol diglycidyl ether). Brit J Cancer 18:407-418, 1964
58. Owen LN, Bostock DE: Prophylactic x-irradiation of the lung in canine tumours with particular reference to osteosarcoma. Europ J Cancer 9:747-752, 1973
59. Owen LN, Bostock DE: Preliminary report on the treatment of canine osteosarcoma using the linear accelerator. Vet Rec (Mar 11): 314, 1972
60. Misdorp W, Hart AAM: Some prognostic and epidemiologic factors in canine osteosarcoma. JNCI 62:537-545, 1979
61. Bourne GH: Biochemistry and Physiology of Bone. 2nd ed. New York, Academic Press, 1972
62. Engle CG, Brodey RS: A retrospective study of 395 feline neoplasms. JAAHA 5:21-31, 1969
63. Harcourt RA: Osteogenic sarcoma in the pig. Vet Rec (Aug 11): 159-161, 1973
64. Sullivan DJ: Cartilagenous tumors (chondroma and chondrosarcoma) in animals. Am J Vet Res 21:531-535, 1960
65. Perk K, Hod 1: Ultrastructure of sheep osteosarcoma. Brit Vet J 129:124-126, 1973
66. Reed C, Garman RH: Osteosarcoma in a squirrel monkey. JAVMA 171:976-979, 1977
67. Simon R: Clinical prognostic factors in osteosarcoma. Cancer Treatment Reports 62:193-197, 1978
68. Harmon TP, Morton RS: Osteogenic sarcoma in four siblings. J Bone & Joint Surg 48:493-498, 1966
Share this article / Teilen Sie diesen Artikel
Diese Webseite wurde mit Jimdo erstellt! Jetzt kostenlos registrieren auf https://de.jimdo.com








