
Article added / Artikel hinzugefügt 01.10.2021
Generally Articles and Discussions about Osteosarcoma in Dogs
→ Evaluations of phylogenetic proximity in a group of 67 dogs with
osteosarcoma: a pilot study
Article added / Artikel hinzugefügt 01.10.2021
Generally Articles and Discussions about Osteosarcoma in Dogs
→ Canine Periosteal Osteosarcoma
Images added / Abbildungen hinzugefügt 02.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Pulmonary vessels
New subcategory added / Neue Unterkategorie hinzugefügt 02.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Pulmonary vessels
Images added / Abbildungen hinzugefügt 01.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Heart valvular diseases



Immunohistochemical investigation of cell cycle and apoptosis regulators (Survivin, β-Catenin, P53, Caspase 3) in canine appendicular osteosarcoma

Bongiovanni, Laura et al. “Immunohistochemical Investigation of Cell Cycle and Apoptosis Regulators (Survivin, Β-Catenin, P53, Caspase 3) in Canine Appendicular Osteosarcoma.” BMC Veterinary Research 8 (2012): 78. PMC. Web. 30 Jan. 2017.

Abstract
Background
Osteosarcoma (OSA) represents the most common canine primary bone tumour. Despite several pathways have been investigated so far, few molecules have been identified as prognostic tools or potential therapeutic targets, and there is still the need to find out molecular pathways with specific influence over OSA progression to facilitate earlier prognosis and treatment.
Aims of the present study were to evaluate the immunohistochemical pattern and levels of expression of a panel of molecules (survivin, β-catenin, caspase 3 -inactive and active forms- and p53) involved in cell cycle and apoptosis regulation in canine OSA samples, known to be of interest in the study also of human OSA, and to detect specific relations among them and with histological tumour grade, disease free interval (DFI) and overall survival (OS).
Results
Nuclear β-catenin immunostaining was detected in normal osteoblasts adjacent to the tumour, and in 47% of the cases. Cytoplasmic and/or membranous immunostaining were also observed. Nuclear survivin and p53 positive cells were found in all cases. Moderate/high cytoplasmic β-catenin expression (≥10% positive cells) was significantly associated with the development of metastasis (P = 0.014); moderate/high nuclear p53 expression (≥10% positive cells) was significantly associated with moderate/high histological grade (P = 0.017) and shorter OS (P = 0.049). Moderate/high nuclear survivin expression (≥15% positive cells) showed a tendency toward a longer OS (P = 0,088).
Conclusions
The present results confirmed p53 as negative prognostic marker, while suggested survivin as a potential positive prognostic indicator, rather than indicative of a poor prognosis. The detection of nuclear β-catenin immunostaining in normal osteoblasts and the absent/low expression in most of the OSAs, suggested that this pathway could not play a major role in oncogenic transformation of canine osteoblasts. Further studies are needed to confirm these hypotheses.
Background
Osteosarcoma (OSA) represents the most common primary bone tumour of both dog [1] and childhood/adolescence [2]. Numerous histological and biological features have been shown to be shared by canine and human OSA to date [3-8], even if further studies are warranted to define more precisely similarities and differences of OSA in the two species, so as to really consider the dog as a valuable spontaneous tumour model for human OSA. Even if several pathways have been investigated in canine OSA so far, few molecules have been identified as prognostic tools or potential therapeutic targets [5,9-18]. Leading on from this, there is still the need to investigate molecular pathways with specific influence over canine OSA progression to facilitate earlier prognosis and treatment.
The goal of the present study was to evaluate a panel of molecules involved in cell cycle and apoptosis regulation in canine OSA samples, known to be of interest in the study also of human OSA. Among these molecules, of particular significance, the role of β-catenin, and the related canonical Wnt pathway, has been largely investigated in human OSA [19-26], but its exact role during neoplastic transformation of osteoblast and OSA progression still remains a debated item. While several studies indicated an important contribution of the activation of the Wnt pathway in OSA development [19-25,27,28], Cai et al. [26] have recently argued the opposite, suggesting that the loss of Wnt pathway activity, which is required for osteoblast differentiation, may contribute to OSA development. Consequently, in contrast to other tumors, β-catenin might not play an oncogenic role in OSA cells [26,29].
Survivin has been recently proposed as a prognostic marker in human [30-35] and canine [36] OSA, but contrasting results have been observed concerning the relation between its subcellular localization and a favourable or poor prognosis. One of the most important aspects of the study of survivin expression in cancer is related to its high cancer specificity and consequent potential role as therapeutic target [37,38]. Survivin has been identified as a target gene of the β-catenin pathway [39], and it acts as a cell cycle regulator and apoptosis inhibitor, even if different isoforms with different functions of the molecule have been described [40].
Loss of p53 tumour suppressor gene functions have been frequently reported in OSA, and mutated p53 has been reported in both human and canine OSA [41]. An interaction between p53 and β-catenin pathway has been proposed to play a key role in osteoblast differentiation and maintenance of bone tissue homeostasis [42]. Furthermore, activation of the Wnt/β-catenin signalling has been shown to cause p53 accumulation [43].
The aims of the present study were to evaluate the immunohistochemical pattern and levels of expression of survivin, β-catenin, caspase 3 (inactive [procaspase 3] and active forms), and p53 in canine OSA samples, to reveal specific relations among them and with histological tumour grade, disease free interval (DFI) and overall survival (OS). The attempt is to contribute to a better understanding of both the pathogenesis of canine OSA and the role of the investigated molecules, in particular establishing their role as prognostic biomarkers and/or potential therapeutic targets. Interestingly, in this study, the expression of nuclear survivin was mainly found in less malignant OSA cases, and β-catenin did not seem to play a major role in oncogenic transformation of canine osteoblasts, while nuclear p53 was confirmed to represent a negative prognostic marker.
Results
Histological examination
OSAs were classified according to WHO classification [44], as: 5 productive osteoblastic, 2 non productive osteoblastic, 1 telengectatic, 2 mixed, 3 fibroblastic, 1 undifferentiated, 1 chondroblastic, and 2 giant cell OSA. According to the Kirpensteijn’s grading system [45], 4 cases were classified as grade I, 7 as grade II, and 6 as grade III OSA (Table 1).
Table 1: Patients data. Tumour grade, breed, sex, age, localization, histological type, post-surgical treatment, DFI, overall survival and development of metastasis of the cases included in the present study.



-catenin expression in canine OSA
In normal osteoblasts surrounding bone trabeculae adjacent to the tumours, β-catenin showed an intense nuclear expression, and a less intense cytoplasmic-membranous expression (Figure 1,a). In tumour cells, membranous β-catenin immunostaining was observed in 65% of the cases, inconsistent and multifocal (Figure 1,b), observed in 2/4 grade I, 5/7 grade II and 4/6 grade III cases. Cytoplasmic staining was found in all the samples examinated (Figure 1, c). Nuclear localization was found in only 47% of the cases, observed in 2/4 grade I, 5/7 grade II and 1/6 grade III cases. The number of positive nuclei were very low: 50% grade I showed no positive nuclei and 50% a number of positive nuclei ranging from 0 to 10%; >0 and <10% positive nuclei were found in 5/7 of grade II; while 5/6 grade III did not show any positive nuclei (Tables 2 and and3).3). Most of the cases showing positive nuclei were fibroblastic OSA (Figure 1, d) (3 cases) or positive nuclei were found in spindle shaped cells present in the case of undifferentiated OSA, and in two cases of productive OSA. Positive nuclei were not found among giant cells, nor among cells of neoplastic emboli, where only cytoplasmic immunostaining was observed (Figure 2, a).
Figure 1: Immunohistochemical β-catenin expression. a – Osteoblasts lining bone trabeculae surrounding neoplastic tissue. Intense nuclear immunostaining (arrows) (Bar. 40 μm) b – Non-productive osteoblastic OSA. Multifocal and inconsistent membranous immunostaining (arrows) (Bar. 80 μm). c - Productive osteoblastic OSA. Diffuse cytoplasmic immunostaining (Bar. 40 μm). d – Fibroblastic OSA. Multifocal nuclear immunostaining (arrows) (Bar. 80 μm).

Table 2: Immunohistochemical results 1. Positive samples for each molecules investigated grouped in different grades, according to Kirpensteijn’s grading system [45].

Table 3: Immunohistochemical results 2. Number of positive cases (and percentage) for each ranges of positive cells observed, grouped in different grades, according to Kirpensteijn’s grading system [45]. N: nuclear; M: membranous; C: cytoplasmic.

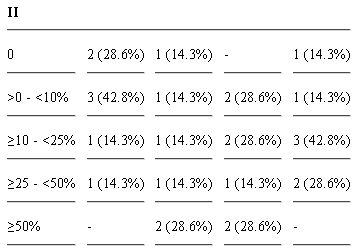

Figure 2: Immunohistochemistry in neoplastic emboli. a – Numerous p53-positive nuclei (arrows) (Bar. 160 μm). b – Weak cytoplasmic and multifocal nuclear (arrows) survivin immunostaining (Bar. 80 μm). c – Weak β-catenin cytoplasmic and no nuclear and membranous immunostaining (Bar. 80 μm).

By Fisher’s exact test moderate/high cytoplasmic β-catenin expression (≥10% positive cells) in the primary tumours was predictive of development of metastasis (P = 0.014). No relations were found between the semi-quantitative evaluation of β-catenin and the OS by Kaplan-Meier survival analysis.
Survivin expression in canine OSA
No staining was found in normal osteoblasts surrounding bone trabeculae adjacent to the tumours, while all samples investigated showed both nuclear and cytoplasmic survivin immunostaining. Cytoplasmic survivin expression was very high (≥75% positive cells) in all the cases evaluated. Nuclear staining was more intense than cytoplasmic, with positive mitotic figures observed among all the tumours (Figure 3, a). Positive nuclei were also multifocally and inconsistently found in the giant cells. Numerous positive nuclei were present among cells of neoplastic emboli (Figure 2, b).
Figure 3: Survivin and p53 immunohistochemical expression. a – Productive osteoblastic OSA. Multifocal survivin nuclear immunostaining(arrows) (Bar. 80 μm). b – Productive osteoblastic OSA. Numerous p53 positive nuclei (arrows) (Bar. 40 μm).

Survivin nuclear expression ranged from 55.55% to 3.86%, with a mean value within the three different groups of 38.22% ± 17.5 (grade I), 16.56% ± 7.9 (grade II) and 13.59% ± 9 (grade III) (mean value ± standard deviation). Statistically significant correlation, using Pearson’s correlation coefficient, was observed between nuclear survivin expression and active caspase 3 expression (ρ = 0.696; P = 0.008), longer DFI (ρ = 0.741; P = 0.001) and OS (ρ = 0.748; P = 0.001); the relation between nuclear survivin expression and active caspase 3 expression seems to be confirmed by the Kendall’s Taub non parametric test (τb = 0.513; P = 0.015), while the same test gives less significant results with longer DFI (P = 0.177) and OS (P = 0.150). The Kaplan-Meier analysis and Log-Rank test (P = 0.088) (Figure 4, a) showed a tendency toward a longer OS in subject with moderate/high nuclear survivin expression (≥15% positive cells).
Figure 4: Kaplan-Meier plots showing influence on survival of survivin and p53. (a) Nuclear survivin expression score ≥15% showed a tendency toward a longer survival time (P = 0,088). (b) P53 nuclear expression score ≥10% appeared to be significantly associated to a longer post-surgical OS (P = 0.049).

p53 expression in canine OSA
Positive nuclei were observed in 82% of cases, found in 2/4 grade I, 6/7 grade II and 6/6 grade III cases (Tables 2 and and3).3). Most of giant cells were negative, and scattered positive nucleoli were also observed (Figure 3, b). Numerous cells within neoplastic emboli showed positive nuclei (Figure 2, c).
A high nuclear p53 expression (≥25% positive cells) was significantly associated with a high histological grade (III) (P = 0.035), while moderate/high nuclear expression (≥10% positive cells) was significantly associated with a moderate/high histological grade (II and III) (P = 0.017) and longer OS (P = 0.004). The Kaplan-Meier analysis and Log-Rank test (P = 0.049) also showed longer OS in subject having moderate/high nuclear p53 expression (≥10% positive cells) (Figure 4, b).
Caspase 3 expression in canine OSA
A diffuse procaspase 3 cytoplasmic immunostaining was found in all the cases investigated; while multifocal active caspase 3-positive nuclei were found in all the neoplastic tissues, but none in normal osteoblasts surrounding bone trabeculae adjacent to the tumours.
Active caspase 3 expression ranged from 65.41% to 5.48%, with a mean value within the three different groups of 53.82% ± 16.4 (grade I), 30.41% ± 23.4 (grade II) and 13.78% ± 14.4 (grade III) (mean value ± standard deviation).
No relations were found between the semi-quantitative evaluation of procaspase 3 and active caspase 3 and the investigated clinico-pathological parameters.
Discussion
The present study demonstrated and confirmed the expression of several important molecules in canine OSA, showing a potential prognostic value of some of them, that could also be considered as possible candidates for a therapeutic target.
Even though the importance of the Wnt/β-catenin pathway in bone development and homeostasis is well defined [46,47], the role of β-catenin in the development and progression of bone tumours is not completely known [48]. Since OSA formation is probably the result of an altered bone regulation, it seems likely that deregulation of the Wnt signalling could be associated with the pathogenesis of OSA. The presence of intense β-catenin-positive nuclei in normal osteoblasts observed in the present study would confirm the fundamental role of the Wnt/β-catenin pathway in the maintenance of these cells. Indeed, nuclear β-catenin expression has been considered a hallmark of activation of the Wnt/β-catenin pathway [26,49] or mutations of β-catenin or molecules of its degradation complex [50]. Rare or completely absent positive nuclei were observed in the most malignant cases examinated herein, even if no significant association with the OS was observed, as well as in neoplastic emboli, suggesting that neither mutations nor activation of the Wnt/β-catenin pathway would occur in these cells. In accordance with that, no mutation has been recently identified in canine β-catenin exon 3, similarly to human OSA [19], even if a limited number of cases were examinated, and a mainly cytoplasmic, but rare nuclear localization of the protein was detected [51]. Furthermore, no differences in nuclear β-catenin immunohistochemical expression were observed in samples obtained from two populations of canine OSA patients with normal or high level of serum alkaline phosphatase concentration [52], known to have negative prognostic value in canine osteosarcoma patients [53]. These results seem to be in agreement with recent investigations in human OSA [26], where no β-catenin nuclear expression was observed in 90% of human high grade OSA biopsies examinated and inhibition of the Wnt/β-catenin pathway has been demonstrated to be important in osteoblast neoplastic transformation [26]. These observations seemed to confirm the hypothesis that inactivation of the Wnt/β-catenin pathway could be essential in OSA progression, differently to what is usually observed in other cancers [54], opening to new potential therapeutic strategies. Specific small molecule compounds that activate Wnt/β-catenin signaling in a highly cell-type specific manner (human OSA U2OS cell line) have recently been identified [55], that could induce the activation of the Wnt/β-catenin pathway and provide new therapeutic opportunities. Interestingly, high β-catenin cytoplasmic expression rate was significantly related to the development of metastasis. High levels of cytoplasmic protein, in absence of nuclear staining, could be related to a reduced membranous β-catenin, reported to be associated to invasion and metastatic potential in human OSA [21], however, an inhibition of β-catenin nuclear translocation may also be hypothesized.
Similarly to what has previously been observed in human [30,31] and canine [36] OSA, both cytoplasmic and nuclear survivin expression pattern was observed in canine OSA. Mechanisms controlling survivin nuclear and/or cytoplasmic localization in tumour cells are often a debated question, since the item could acquire a different prognostic significance depending on the tumour type [56]. Our results seem to be in contrast with a recent study in which a correlation between survivin immunohistochemical expression and histological grade and mitotic index has been reported in canine OSA tissue samples [36]. Similarly, divergent results have been published concerning the relation between survivin expression and malignancy of human OSA [30,32-34]. Disagreements could be related to the presence of several survivin isoforms, with different, even opposite, functions, and this might indicate different activities of survivin within the cell. In particular, two survivin variants potentially act in an opposite way to the other three variants, with a potential proapoptotic function: Survivin-2α may attenuate the anti-apoptotic activity of full length survivin [57], while survivin-deltaEx3 has been shown to have apoptotic functions [58]. Noteworthy, the polyclonal antibody used in the present study recognizes all survivin splice variants, and decreased nuclear survivin expression score was found among more malignant grade III cases of canine OSA, as well as higher nuclear survivin expression appeared to be related to a longer post-treatment OS, similarly to that observed by Trieb et al. [30] in the human counterpart. A significant association was revealed between nuclear survivin expression and the activation of caspase 3, most probably indicative of apoptotic pathway activation, further supporting the hypothesis that nuclear survivin expression could be considered a positive prognostic marker in canine OSA. Indeed, despite a diffuse cytoplasmic staining of its inactive form (procaspase 3) observed in all the cases examinated, a progressive decreasing of the active caspase 3 mean values was observed in OSA from grade I to III.
Differently to primary tumours, a frequent nuclear survivin immunostaining was observed among neoplastic emboli, suggesting a different behaviour of the protein in OSA metastatic spread. Therefore, further investigations on survivin expression in metastatic OSA are warranted, in order to understand its functions and role in the response to postoperative chemotherapy, since adjuvant treatment is mainly aimed at controlling the metastatic disease, and survivin has been frequently associated to resistance to chemotherapy [59,60]. Recently, this has been demonstrated also in canine OSA cell lines (Abrams and D17), where after survivin inhibition an increase of chemosensitivity to both doxorubicin and carboplatin has been observed [36]. Survivin has been proposed as a valuable target for anticancer therapy [37], and several molecules with a direct or indirect effect on survivin expression have been identified to date, some of which have been tested in clinical trials with encouraging results [61]. Decreasing survivin expression through STAT3 [5,62] or Hsp90 [63] inhibition in canine OSA cell lines has been recently proposed as a possible new therapeutic approach.
There is a strong evidence of the involvement of p53 mutations in the development of canine OSA [41,64,65], as well as in the human counterpart [66,67]. In the present study, highest levels of expression of nuclear p53 were significantly associated with a shorter post surgical OS, and appeared to be related to the development of metastasis, confirming the importance of p53 as a negative prognostic marker in canine OSA, in accordance to previous published data regarding canine OSA, suggesting that, similarly to human OSA [68,69], alterations in p53 functions are associated with highly aggressive tumour behaviour [70,71]. Deregulation of the p53-survivin subsystem has been proposed to play an important role in several tumour types [72], since, WTp53 is able to repress survivin expression at both the mRNA and protein level [40]. In spite of this, our results suggests that different mechanisms could regulate survivin expression in canine OSA.
Conclusions
Data obtained from the present immunohistochemical study suggest survivin as a potential positive prognostic marker, rather than a marker of poor prognosis, even if its presence in metastatic emboli suggests its possible involvement in the malignant progression of canine OSA. Further investigations, in particular on the expression and roles of its different isoforms, should be performed in order to clarify if survivin could represent a valid biomarker in this kind of tumour.
Lastly, the present data suggest that β-catenin, a key molecule for normal osteoblast differentiation, could not play a key role in the neoplastic transformation of canine osteoblasts, as recently suggested in human OSA. Further studies should be done in order to confirm this hypothesis.
Methods
Tissue Samples and Clinical data
This retrospective study looked at of 17 canine OSA surgical samples, provided by the Department of Animal Pathology, Faculty of Veterinary Medicine, University of Torino (Italy), for which survival data were also provided. All the dogs included in the present study had an appendicular OSA (histologically confirmed) at presentation and no evidence of metastasis. Clinical staging included history, physical exam, complete blood count, serum biochemical profile, urinalysis and abdominal ultrasound. Limb (latero-lateral [LL] and antero-posterior [AP] views) and chest (right and left LL, and dorso-ventral [DV] views) radiographic evaluation was performed to examine features and extension of the tumour and presence of lung metastasis, respectively. Computed Tomography was performed in case of radiographic suspicion of lung metastasis. Regional lymph nodes were aspirated and cytologically examined when enlarged at clinical palpation. Initial diagnosis of the tumour was attempted by fine needle aspiration and cytology but, for a more specific identification of the tumour type, a preoperative biopsy was obtained in all cases using a Jamshidi needle and submitted to histopathology. Histopathology was performed also on the entire tumour specimen (in case of limb amputation or limb sparing using an allograft to substitute the tumour segment) or on a sample of the tumour (in case of limb sparing using the pasteurized neoplastic autograft), in order to confirm the diagnosis [73,74]. All dogs included in the present study were surgically treated (amputation or limb sparing) before receiving adjuvant chemotherapy using doxorubicin (30 mg/m2, 4–5 administrations, 21 days apart) or cisplatin (70 mg/m2, 4–5 administrations, 21 days apart) as a single agent, or a combination of cisplatin and doxorubicin (4 cycles, 21 days apart, each cycle consisting of cisplatin 50 mg/m2 at day 1 and doxorubicin 15 mg/m2 at day 2). One of the three chemotherapy protocols was chosen based on the dog clinical status and owners compliance, since no protocol has been demonstrated to be superior [75-78]. Besides, histopathological tumour grade never influenced the choice of the chemotherapeutic protocol adopted. Canine patients were clinically and radiographically examined every 3 months during the first year after the conclusion of chemotherapy and then every 6 months for a minimum of 2 years. DFI (disease free interval) was considered as the period in days from surgery to recurrence and/or metastasis development, while OS (overall survival) as the period in days from surgery to death for tumour-related causes; for dogs still alive at the time of writing, OS was the number of days from surgery to the last clinical examination (patients’ data are summarized in Table 1).
Histological examination
All specimens were routinely fixed in 10% formalin, embedded in paraffin wax, and 4–5 μm-thick sections were examined using haematoxylin and eosin (H&E) staining and visualized by light microscopy. Tumours were histologically classified according to the World Health Organization (WHO) criteria [44], and histological grade was determined according to the system proposed by Kirpensteijn et al. [45].
Immunohistochemical Examination
Dewaxed and rehydrated tissue sections were immunostained by the streptavidin-biotin peroxidase complex (SAB) method, using specific primary antibodies (Abs) reported in Table 4, incubated overnight in a humidified chamber at 4°C. Immunohistochemical analysis followed procedure as reported in previous studies [79]. Endogenous peroxidase was blocked with H2O2 3% in absolute methanol for 45 min. Antigen retrieval was undertaken by heat-treating sections in citrate buffer at pH 6 (β-catenin, caspase 3) or Tris-EDTA pH 9.0 (p53) in a microwave oven for 5min. (3 cycles) or in citrate buffer at pH 6 in a pressure cooker for 20 min. (survivin). Presence of antibody binding was visualized with 3-3’-diaminobenzidine (DAB, D5905, Sigma-Aldrich, St. Louis, MO, USA) solution, which was applied for 5 min, followed by a light counterstain with Mayer’s haematoxylin (Merck, Darmstadt, Germany) for 1 min. Positive control slides were used for each Abs using a tissue of known pattern of expression of each molecules (Table 4). Anti-p53 antibody validation was performed using samples of human mammary carcinoma (Figure 5, a) and a samples of canine nail bed squamous cell carcinoma (SCC) (Figure 5, b) then used as positive control. A negative control was performed in all instances by omitting the primary antibody and incubating tissue sections with Tris Buffered Saline (TBS); for active caspase 3, an irrelevant antibody directed against an unrelated antigen (mouse anti human desmin monoclonal antibody – Dako, Glostrub, Denmark) was also used.
Table 4: Primary antibodies used in immunohistochemical analysis. Clonality, company, working dilutions and positive controls used for each antibody. PAb: polyclonal antibody; MAb: monoclonal antibody. SCC: squamous cell carcinoma.

Figure 5: Positive control tissues for p53. a- Human mammary carcinoma. p53-positive nuclei (arrows) (Bar. 40 μm). b – Canine nail bed squamous cell carcinoma. Numerous p53-positive nuclei (arrows) (Bar. 40 μm).

Quantification of Immunostaining and Statistical Analysis
Neoplastic tissues were scored by two pathologists as follow.
Survivin and caspase 3 expression was evaluated by counting the number of positive nuclei in 10 HPF at 400X, counting approximately 1000 cells, and expressed as a percentage. Cytoplasmic survivin expression was diffusely present in 100% of the cases evaluated.
For p53 only nuclear staining was considered as positive, since nuclear immunostaining is suggestive of its mutation [68]; β-catenin expression was classified as membranous (localised at cell-cell boundaries), cytoplasmic (uniformly distributed throughout the cytoplasm) and nuclear. A semi-quantitative immunohistochemical assessment was performed and samples were subdivided based on the protein expression levels in 4 ranges for β-catenin and p53 (absent: no positive cells; low: >0% - <10% positive cells; moderate: ≥10% - <50% positive cells; high: ≥50% positive cells) and 5 ranges for procaspase 3 and survivin (absent: no positive cells; low: >0% - <10% positive cells; moderate: ≥10% - <50% positive cells; high: ≥50% - <75% positive cells; very high ≥75% positive cells).
Fisher's exact test was applied to evaluate the association between the expression levels of the investigated molecules and clinico-pathological parameters. Pearson correlation test and Kendall’s Taub test were applied to examine the relations between the expression levels of nuclear survivin and p53, DFI, OS. Kaplan Meier analysis was used to estimate survival, and the significances of the differences were determined by the Log-Rank test. For this purpose, the cases were grouped according to the expression score as follows: <10% positive cells (absent + low semiquantitative evaluation) versus ≥10% positive cells (moderate + high semiquantitative evaluation) for p53 and β-catenin; <15% positive cells (absent + low semiquantitative evaluation) versus ≥15% positive cells (moderate + high semiquantitative evaluation) for survivin; or <25% positive cells (absent/low + moderate semiquantitative evaluation) versus ≥25% positive cells (high semiquantitative evaluation). Analyses were performed using the IBM SPSS19 statistical software, and the conventional 5% level was used to define statistical significance.
Authors’ contributions
LB participated in the design of the study and drafted the manuscript. FM carried out the immunohistochemistry and helped to draft the manuscript. MD participated in the design of the study. RM participated in the design of the study and helped to draft the manuscript. AC performed the statistical analysis and participate in the interpretation of the results. PB performed surgical treatment and adjuvant chemotherapy, providing follow-up and clinical data of the patients. RDM provided technical support. CP provided technical support. MM performed surgical treatment and adjuvant chemotherapy, providing follow-up and clinical data of the patients. ME performed surgical treatment and adjuvant chemotherapy, providing follow-up and clinical data of the patients. ML provided technical support. LDS conceived of the study, participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgement
This work was supported by a grant (PRIN 2008) from the Italian Ministry of University and Scientific Research, Rome, Italy.
References
- Endicott M. Principles of treatment for osteosarcoma. Clin Tech Small Anim Pract. 2003;18:110–114. doi: 10.1053/svms.2003.36626. [PubMed] [Cross Ref]
- Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115(7):1531–1543. doi: 10.1002/cncr.24121. [PMC free article] [PubMed] [Cross Ref]
- Mueller F, Fuchs B, Kaser-Hotz B. Comparative Biology of Human and Canine Osteosarcoma. Anticancer Res. 2007;27:155–164. [PubMed]
- De Maria R, Miretti S, Iussich S, Olivero M, Morello E, Bertotti A, Christensen JG, Levine RA, Buracco P, Di Renzo MF. Met oncogene activation qualifies spontaneous canine osteosarcoma as a suitable pre-clinical model of human osteosarcoma. J Pathol. 2009;218:399–408. doi: 10.1002/path.2549. [PubMed] [Cross Ref]
- Fossey SL, Liao AT, McCleese JK, Bear MD, Lin J, Li PK, Kisseberth WC, London CA. Characterization of STAT3 activation and expression in canine and human osteosarcoma. BMC Cancer. 2009;9:81. doi: 10.1186/1471-2407-9-81. [PMC free article] [PubMed] [Cross Ref]
- Thomas R, Wang HJ, Tsai PC, Langford CF, Fosmire SP, Jubala CM, Getzy DM, Cutter GR, Modiano JF, Breen M. Influence of genetic background on tumor karyotypes: evidence for breed–asscoiated aberrations in canine appendicular osteosarcoma. Chromosome Res. 2009;17:365–377. doi: 10.1007/s10577-009-9028-z. [PMC free article] [PubMed] [Cross Ref]
- Morello E, Martano M, Buracco P. Biology, diagnosis and treatment of canine appendicular osteosarcoma: Similarities and differences with human osteosarcoma. Vet J. 2011;189(3):268–277. doi: 10.1016/j.tvjl.2010.08.014. [PubMed] [Cross Ref]
- Withrow SJ, Wilkins RM. Cross talk from pets to people: translational osteosarcoma treatments. ILAR J. 2010;51(3):208–213. [PubMed]
- MacEwen EG, Pastor J, Kutzke J, Tsan R, Kurzman ID, Thamm DH, Wilson M, Radinsky R. IGF-1 receptor contributes to the malignant phenotype in human and canine osteosarcoma. J Cell Biochem. 2004;92(1):77–91. doi: 10.1002/jcb.20046. [PubMed] [Cross Ref]
- Flint AF, U'Ren L, Legare ME, Withrow SJ, Dernell W, Hanneman WH. Overexpression of the erbB-2 proto-oncogene in canine osteosarcoma cell lines and tumors. Vet Pathol. 2004;41(3):291–296. doi: 10.1354/vp.41-3-291. [PubMed] [Cross Ref]
- Mullins MN, Lana SE, Dernell WS, Ogilvie GK, Withrow SJ, Ehrhart EJ. Cyclooxygenase-2 expression in canine appendicular osteosarcomas. J Vet Intern Med. 2004;18(6):859–865. doi: 10.1111/j.1939-1676.2004.tb02633.x. [PubMed] [Cross Ref]
- Kow K, Thamm DH, Terry J, Grunerud K, Bailey SM, Withrow SJ, Lana SE. Impact of telomerase status on canine osteosarcoma patients. J Vet Intern Med. 2008;22(6):1366–1372. doi: 10.1111/j.1939-1676.2008.0175.x. [PubMed] [Cross Ref]
- Kirpensteijn J, Kik M, Teske E, Rutteman GR. TP53 gene mutations in canine osteosarcoma. Vet Surg. 2008;37(5):454–460. doi: 10.1111/j.1532-950X.2008.00407.x. [PubMed] [Cross Ref]
- Selvarajah GT, Kirpensteijn J, van Wolferen ME, Rao NA, Fieten H, Mol JA. Gene expression profiling of canine osteosarcoma reveals genes associated with short and long survival times. Mol Cancer. 2009;8:72. doi: 10.1186/1476-4598-8-72. [PMC free article] [PubMed] [Cross Ref]
- Selvarajah GT, Kirpensteijn J. Prognostic and predictive biomarkers of canine osteosarcoma. Vet J. 2010;185(1):28–35. doi: 10.1016/j.tvjl.2010.04.010. [PubMed] [Cross Ref]
- O'Donoghue LE, Ptitsyn AA, Kamstock DA, Siebert J, Thomas RS, Duval DL. Expression profiling in canine osteosarcoma: identification of biomarkers and pathways associated with outcome. BMC Cancer. 2010;10:506. doi: 10.1186/1471-2407-10-506. [PMC free article] [PubMed] [Cross Ref]
- Sharili AS, Allen S, Smith K, Hargreaves J, Price J, McGonnell I. Expression of Snail2 in long bone osteosarcomas correlates with tumour malignancy. Tumour Biol. 2011;32(3):515–526. doi: 10.1007/s13277-010-0146-1. [PMC free article] [PubMed] [Cross Ref]
- Romanucci M, D’Amato G, Malatesta D, Bongiovanni L, Palmieri C, Ciccarelli A, Buracco P, Morello E, Maniscalco L, De Maria R, Martano M, Della Salda L. Heat shock protein expression in canine osteosarcoma. Cell Stress Chaperones. 2012;17(1)):131–138. [PMC free article] [PubMed]
- Haydon RC, Deyrup A, Ishikawa A, Heck R, Jiang W, Zhou L, Feng T, King D, Cheng H, Breyer B, Peabody T, Simon MA, Montag AG, He TC. Cytoplasmic and/or nuclear accumulation of the beta-catenin protein is a frequent event in human osteosarcoma. Int J Cancer. 2002;102(4):338–342. doi: 10.1002/ijc.10719. [PMC free article] [PubMed] [Cross Ref]
- Iwaya K, Ogawa H, Kuroda M, Izumi M, Ishida T, Mukay K. Cytoplasmatic and/or nuclear staining of beta-catenin is associated with lung metastasis. Clin Exp Metastasis. 2003;20:525–529. doi: 10.1023/A:1025821229013. [PubMed] [Cross Ref]
- Kashima T, Kawaguchi J, Takeshita S, Kuroda M, Takanashi M, Horiuchi H, Imamura T, Ishikawa Y, Ishida T, Mori S, Machinami R, Kudo A. Anomalous cadherin expression in osteosarcoma, Possible relationships to metastasis and morphogenesis. Am J Pathol. 1999;155(5):1549–1555. doi: 10.1016/S0002-9440(10)65471-5. [PMC free article] [PubMed] [Cross Ref]
- Kashima T, Nakamura K, Kawaguchi J, Takanashi M, Ishida T, Aburatani H, Kudo A, Fukayama M, Grigoriadis AE. Overexpression of cadherins suppresses pulmonary metastasis of osteosarcoma in vivo. Int J Cancer. 2003;104:147–154. doi: 10.1002/ijc.10931. [PubMed] [Cross Ref]
- Chen K, Fallen S, Abaan HO, Hayran M, Gonzalez C, Wodajo F, MacDonald T, Toretsky JA, Uren A. Wnt 10 b induces chemotaxis of osteosarcoma and correlates with reduced survival. Pediatr Blood Cancer. 2008;51:349–355. doi: 10.1002/pbc.21595. [PubMed] [Cross Ref]
- Clenton-Jansen AM, Anninga JK, Briaire-de Bruijn IH, Romeo S, Oosting J, Egeler RM, Gelderblom H, Taminiau AH, Hogendoorn PC. Profiling 78 of high grade central osteosarcoma and its putative progenitor cells identifies tumorigenic pathways. Br J Cancer. 2009;101:1909–1918. doi: 10.1038/sj.bjc.6605405. [PMC free article] [PubMed] [Cross Ref]
- Leow PC, Tian Q, Ong ZY, Yang Z, Ee PL. Antitumor activity of natural compounds, curcumin and PKF 118–310, as Wnt/beta-catenin antagonists against human osteosarcoma cells. Invest New Drugs. 2010;28(6):766–782. doi: 10.1007/s10637-009-9311-z. [PubMed] [Cross Ref]
- Cai Y, Mohseny AB, Karperien M, Hogendoorn PC, Zhou G, Cleton-Jansen AM. Inactive Wnt/beta-catenin pathway in conventional high-grade osteosarcoma. J Pathol. 2010;220(1):24–33. doi: 10.1002/path.2628. [PubMed] [Cross Ref]
- Hoang BH, Kubo T, Healey JH, Sowers R, Mazza B, Yang R, Huvos AG, Meyers PA, Gorlick R. Expression of LDL-receptors-related protein 5 (LRP5) as a novel marker for disease progression in high-grade osteosarcoma. Int J Cancer. 2004;109:106–111. doi: 10.1002/ijc.11677. [PubMed] [Cross Ref]
- Kansara M, Tsang M, Kodjabachian L, Sims NA, Trivett MK, Ehrich M, Dobrovic A, Slavin J, Choong PF, Simmons PJ, Dawid IB, Thomas MD. Wnt inhibitory factor 1 is epigenetically silenced in human osteosarcoma, and targeted distruption accelerates osteosarcomagenesis in mice. J Clin Invest. 2009;119:837–851. doi: 10.1172/JCI37175. [PMC free article] [PubMed] [Cross Ref]
- Zhang F, Chen A, Chen J, Yu T, Guo F. Influence of β-catenin small interfering RNA on human osteosarcoma cells. J Huazhong Univ Sci Technolog Med Sci. 2011;31(3):353–358. doi: 10.1007/s11596-011-0380-9. [PubMed] [Cross Ref]
- Trieb K, Lehner R, Stulnig R, Sulzbacher I, Shoryer KR. Survivin expression in human osteosarcoma is amarker for survivalEur. J Surg Oncol. 2003;29:379–382. doi: 10.1053/ejso.2002.1415. [PubMed] [Cross Ref]
- Wang W, Huiying L, Wang A. Expression of Survivin and correlation with PCNA in Osteosarcoma. J Surg Oncol. 2006;93:578–584. doi: 10.1002/jso.20507. [PubMed] [Cross Ref]
- Osaka E, Suzuki T, Osaka S, Yoshida Y, Sugita H, Asami S, Tabata K, Hemmi A, Sugitani M, Nemoto N, Ryu J. Survivin as a prognostic factor for osteosarcoma patients. JSHC. 2006;39(3):95–100. [PMC free article] [PubMed]
- Osaka E, Suzuki T, Osaka S, Yoshida Y, Sugita H, Asami S, Tabata K, Hemmi A, Sugitani M, Nemoto N, Ryu J. Survivin expression levels as Indipendent predictors of survival for osteosarcoma patients. J Orthop Res. 2007;25(1):116–121. doi: 10.1002/jor.20291. [PubMed] [Cross Ref]
- Clark JC, Dass CR, Choong PF. A review of clinical and molecular prognostic factors in osteosarcoma. J Cancer Res Clin Oncol. 2008;134(3):281–297. doi: 10.1007/s00432-007-0330-x. [PubMed] [Cross Ref]
- Do SI, Jung WW, Kim HS, Park Y. The expression of epidermal growth factor receptor and its downstream signaling molecules in osteosarcoma. Inter Journal of oncol. 2009;34:797–803. [PubMed]
- Shoeneman JK, Ehrhart EJ 3rd, Eickhoff JC, Charles JB, Powers BE, Thamm DH. Expression and function of survivin in canine osteosarcoma. Cancer Res. 2012;72(1)):249–259. [PubMed]
- Kanwar JR, Kamalapuram SK, Kanwar RK. Targeting survivin in cancer: the cell-signalling perspective. Drug Discov Today. 2011;16(11–12):485–494. [PubMed]
- Guha M, Altieri DC. Survivin as a global target of intrinsic tumor suppression networks. Cell Cycle. 2009;8(17):2708–2710. doi: 10.4161/cc.8.17.9457. [PMC free article] [PubMed] [Cross Ref]
- Kim PJ, Plescia J, Clevers H, Fearon ER, Altieri DC. Survivin and molecular pathogenesis of colorectal cancer. Lancet. 2003;362(9379):205–209. doi: 10.1016/S0140-6736(03)13910-4. [PubMed] [Cross Ref]
- Bongiovanni L, Muller EJ, Della Salda L. Survivin in skin pathologies. Exp Dermatol. 2011;20(6):456–463. [PubMed]
- Mendoza S, Conischi T, Dernell WS, Withrow SJ, Miller CW. Status of the p53, Rb and Mdm2 Genes in Canine Osteosarcoma. Anticancer Res. 1998;18:4449–4454. [PubMed]
- Chandar N, Saluja R, Lamar PC, Kolman K, Prozialeck WC. P53 and Beta-catenin activity during estrogen treatment of osteoblasts. Cancer Cell International. 2005;5:24. doi: 10.1186/1475-2867-5-24. [PMC free article] [PubMed] [Cross Ref]
- Damalas A, Kahan S, Shtutman M, Ben-Ze’ev A, Oren M. Deregulated β-catenin induces a p53- and ARF-dependent growth arrest and cooperated with Ras in transformation. EMBO J. 2001;20(17):4912–4922. doi: 10.1093/emboj/20.17.4912. [PMC free article] [PubMed] [Cross Ref]
- Slayter MV, Boosinger TR, Pool RR, Dammrich K, Misdorp W, Larsen S. Histological classification of bone and joint tumors of domestic animals, Vol.1. 2. Armed Forces Institute of Pathology, Washington DC; 1994. pp. 5–45.
- Kirpensteijn J, Kik M, Rutteman GR, Teske E. Prognostic significance of a new histologic grading system for Canine Osteosarcoma. Vet Pathol. 2002;39(2):240–246. doi: 10.1354/vp.39-2-240. [PubMed] [Cross Ref]
- Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8(5):739–750. doi: 10.1016/j.devcel.2005.03.016. [PubMed] [Cross Ref]
- Glass DA 2nd, Karsenty G. In vivo analysis of Wnt signaling in bone. Endocrinology. 2007;148(6):2630–2634. doi: 10.1210/en.2006-1372. [PubMed] [Cross Ref]
- Thomas DM. Wnts, bone and cancer. J Pathol. 2010;220(1):1–4. doi: 10.1002/path.2635. [PubMed] [Cross Ref]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [PubMed] [Cross Ref]
- Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19(2):150–158. doi: 10.1016/j.ceb.2007.02.007. [PubMed] [Cross Ref]
- Stein TJ, Holmes KE, Muthuswamy A, Thompson V, Huelsmeyer MK. Characterization of β-catenin expression in canine osteosarcoma. Vet Comp Oncol. 2011;9(1):65–73. doi: 10.1111/j.1476-5829.2010.00236.x. [PMC free article] [PubMed] [Cross Ref]
- Piskun CM, Muthuswamy A, Huelsmeyer MK, Thompson V, Stein TJ. Wnt/β-catenin expression does not correlate with serum alkaline phosphatase concentration in canine osteosarcoma patients. PLoS One. 2011;6(10):e26106. doi: 10.1371/journal.pone.0026106. [PMC free article] [PubMed] [Cross Ref]
- Garzotto CK, Berg J, Hoffmann WE, Rand WM. Prognostic significance of serum alkaline phosphatase activity in canine appendicular osteosarcoma. J Vet Int Med. 2000;14:587–592. doi: 10.1111/j.1939-1676.2000.tb02281.x. [PubMed] [Cross Ref]
- Behrens J, Lustig B. The Wnt connection to tumorigenesis. Int J Dev Biol. 2004;48:477–487. doi: 10.1387/ijdb.041815jb. [PubMed] [Cross Ref]
- Verkaar F, van der Stelt M, Blankesteijn WM, van der Doelen AA, Zaman GJ. Discovery of novel small molecule activators of β-catenin signaling. PLoS One. 2011;6(4):e19185. doi: 10.1371/journal.pone.0019185. [PMC free article] [PubMed] [Cross Ref]
- Stauber RH, Mann W, Knauer SK. Nuclear and cytoplasmic survivin: molecular mechanism, prognostic, and therapeutic potential. Cancer Res. 2007;67(13):5999–6002. doi: 10.1158/0008-5472.CAN-07-0494. [PubMed] [Cross Ref]
- Caldas H, Honsey LE, Altura RA. Survivin 2alpha: a novel Survivin splice variant expressed in human malignancies. Mol Cancer. 2005;4:11. doi: 10.1186/1476-4598-4-11. [PMC free article] [PubMed] [Cross Ref]
- Mahotka C, Wenzel M, Springer E, Gabbert HE, Gerharz CD. Survivin-deltaEx3 and survivin-2B: two novel splice variants of the apoptosis inhibitor survivin with different antiapoptotic properties. Cancer Res. 1999;59:6097–6102. [PubMed]
- Su L, Wang Y, Xiao M, Lin Y, Yu L. Up-regulation of survivin in oral squamous cell carcinoma correlates with poor prognosis and chemoresistance. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(4):484–491. doi: 10.1016/j.tripleo.2010.04.009. [PubMed] [Cross Ref]
- Lechler P, Renkawitz T, Campean V, Balakrishnan S, Tingart M, Grifka J, Schaumburger J. The antiapoptotic gene survivin is highly expressed in human chondrosarcoma and promotes drug resistance in chondrosarcoma cells in vitro. BMC Cancer. 2011;11:120. doi: 10.1186/1471-2407-11-120. [PMC free article] [PubMed] [Cross Ref]
- Cheung CH, Cheng L, Chang KY, Chen HH, Chang JY. Investigations of survivin: the past, present and future. Front Biosci. 2011;16:952–961. doi: 10.2741/3728. [PubMed] [Cross Ref]
- Fossey SL, Bear MD, Kisseberth WC, Pennell M, London CA. Oncostatin M promotes STAT3 activation, VEGF production, and invasion in osteosarcoma cell lines. BMC Cancer. 2011;11:125. doi: 10.1186/1471-2407-11-125. [PMC free article] [PubMed] [Cross Ref]
- McCleese JK, Bear MD, Fossey SL, Mihalek RM, Foley KP, Ying W, Barsoum J, London CA. The novel HSP90 inhibitor STA-1474 exhibits biologic activity against osteosarcoma cell lines. Int J Cancer. 2009;125(12):2792–2801. doi: 10.1002/ijc.24660. [PubMed] [Cross Ref]
- Van Leeuwen IS, Cornelisse CJ, Misdorp W, Goedegebuure SA, Kirpensteijn J, Rutteman GR. p53 gene mutations in osteosarcoma in the dog. Cancer Lett. 1997;111:173–178. doi: 10.1016/S0304-3835(96)04529-6. [PubMed] [Cross Ref]
- Johnson AS, Couto CG, Weghorst CM. Mutations of p53 tumor suppressor gene in spontaneously occurring osteosarcomas of the dog. Carcinogenesis. 1998;19:213–217. doi: 10.1093/carcin/19.1.213. [PubMed] [Cross Ref]
- Miller CW, Aslo A, Tsay C, Slamon D, Ishizaki K, Toguchida J, Yamamuro T, Lampkin B, Koeffler HP. Frequency and structure of p53 rearrangements in human osteosarcoma. Cancer Res. 1990;50(24):7950–7954. [PubMed]
- Tang N, Song WX, Luo J, Haydon RC, He TC. Osteosarcoma and stem cell Differentiation. Clin Orthop Relat Res. 2008;466:2114–2130. doi: 10.1007/s11999-008-0335-z. [PMC free article] [PubMed] [Cross Ref]
- Ueda Y, Dockhorn-Dworniczak B, Blasius S, Mellin W, Wuisman P, Werner B, Roessner A. Analysis of mutant p53 protein in osteosarcomas and other malignant and beign lesions of bone. J Cancer Res Clin Oncol. 1993;119:172–178. doi: 10.1007/BF01229533. [PubMed] [Cross Ref]
- Boulytcheva IV, Soloviev YN, Kushlinskii NE, Mahson AN. Expression of molecular markers in the tumor and survival prognosis in osteosarcoma. Bull Exp Biol Med. 2010;150(2):237–242. doi: 10.1007/s10517-010-1114-x. [PubMed] [Cross Ref]
- Sagartz JE, Bodley WL, Gamblin RM, Couto CG, Tierney LA, Capen CC. p53 tumor suppressor protein overexpression in osteogenic tumors of dogs. Vet Pathol. 1996;33:213–221. doi: 10.1177/030098589603300211. [PubMed] [Cross Ref]
- Loukopoulos P, Thornthon JR, Robison WF. Clinical and Pathologic Relevance of p53 Index in canine osseus tumors. Vet Pathol. 2003;40:237–248. doi: 10.1354/vp.40-3-237. [PubMed] [Cross Ref]
- Altieri DC. New wirings in the survivin networks. Oncogene. 2008;27(48):6276–6284. doi: 10.1038/onc.2008.303. [PMC free article] [PubMed] [Cross Ref]
- Buracco P, Martano M, Morello E, Vasconi ME. Pasteurized tumoral autograft as a novel procedure of limb sparing in dogs: a clinical report in a canine distal radial osteosarcoma. Vet Surg. 2002;31(6):525–532. doi: 10.1053/jvet.2002.34674. [PubMed] [Cross Ref]
- Morello E, Vasconi E, Martano M, Peirone B, Buracco P. Pasteurized Tumoral Autograft and Adjuvant Chemotherapy for the Treatment of Canine Distal Radial Osteosarcoma: 13 cases. Vet Surg. 2003;32(6):539–544. doi: 10.1111/j.1532-950X.2003.00539.x. [PubMed] [Cross Ref]
- Dernell WS, Ehrhart NP, Straw RC, Vail DM. In: Withrow & MacEwen’s Small animal clinical oncology. 4. Withrow SJ, Vail DM, Saunders , editor. Elsevier, St Louis, Missouri; 2007. Tumors of the skeletal system; pp. 540–582.
- Straw RC, Withrow SJ, Richter SL, Powers BE, Klein MK, Postorino NC, LaRue SM, Ogilvie GK, Vail DM, Morrison WB, McGee M, Dickinson. Amputation and cisplatin for treatment of canine osteosarcoma. J Vet Intern Med. 1991;5:205–210. doi: 10.1111/j.1939-1676.1991.tb00950.x. [PubMed] [Cross Ref]
- Berg J, Weinstein MJ, Springfield DS, Rand WM. Results of surgery and doxorubicin chemotherapy in dogs with osteosarcoma. J Am Vet Med Assoc. 1995;206:1555–1560. [PubMed]
- Chun R, Garrett LD, Henry C, Wall M, Smith A, Azene NM. Toxicity and efficacy of cisplatin and doxorubicin combination chemotherapy for the treatment of canine osteosarcoma. J Am Anim Hosp Assoc. 2005;41:382–387. [PubMed]
- Bongiovanni L, Colombi I, Fortunato C, Della Salda L. Survivin expression in canine epidermis and in canine and human cutaneous squamous cell carcinomas. Vet Dermatol. 2009;20(5–6):369–376. [PubMed]
- Bongiovanni L, Malatesta D, Brachelente C, D’Egidio S, Della Salda L. β-Catenin In Canine Skin: Immunohistochemical Pattern Of Expression In Normal Skin And Cutaneous Epithelial Tumors. J Comp Pathol. 2011;145(2–3):138–147. [PubMed]
- Bongiovanni L, Romanucci M, Fant P, Lagadic M, Della Salda L. Apoptosis and anti-apoptotic heat shock proteins in canine cutaneous infundibular keratinizing acanthomas and squamous cell carcinomas. Vet Dermatol. 2008;19(5):271–279. doi: 10.1111/j.1365-3164.2008.00687.x. [PubMed] [Cross Ref]
Share this article / Teilen Sie diesen Artikel
Diese Webseite wurde mit Jimdo erstellt! Jetzt kostenlos registrieren auf https://de.jimdo.com








