Abstract
Background
Tyrosine kinase inhibitors (TKIs) and metronomic dosing of cyclophosphamide (CYC) can improve tumor control by suppression of regulatory T cells (Treg) and restoration of T
cell-mediated immune responses in mice and humans. The immunomodulatory effects of the TKI toceranib, as a single agent or in combination with metronomic CYC, have not been previously
investigated in dogs.
Hypothesis
The primary objectives of this study were to determine the effects of toceranib and metronomic CYC treatment on lymphocyte subsets including Treg and on interferon-gamma (IFN-γ)
secretion in dogs with cancer. We hypothesized that toceranib would selectively decrease Treg numbers and increase IFN-γ production and that addition of CYC would further enhance
these effects.
Animals
Fifteen client-owned dogs with advanced tumors were entered into a prospective clinical trial.
Methods
Dogs received toceranib at 2.75 mg/kg once every other day. After 2 weeks, oral CYC was added at 15 mg/m2 daily. Numbers of Treg and lymphocyte subsets were
measured in blood by flow cytometry during the 8-week study period. Serum concentrations of IFN-γ were measured by ELISA.
Results
Administration of toceranib significantly decreased the number and percentage of Treg in the peripheral blood of dogs with cancer. Dogs receiving toceranib and CYC demonstrated a
significant increase in serum concentrations of IFN-γ, which was inversely correlated with Treg numbers after 6 weeks of combination treatment.
Conclusions
In addition to antitumor effects, these data support further investigations into the immunomodulatory effects of toceranib, administered alone or in combination with CYC in dogs with
cancer.
Abbreviations
-
CYC
-
cyclophosphamide
-
SHC
-
sterile hemorrhagic cystitis
-
Treg
-
regulatory T cells
-
TKI
-
tyrosine kinase inhibitor
Toceranib phosphate1 is a receptor tyrosine kinase inhibitor (TKI) active against the split kinase family of tyrosine kinases including Kit, vascular endothelial growth factor receptor-2,
platelet-derived growth factor receptor, and Flt-3.[1] Toceranib and structurally similar TKIs such as sunitinib demonstrate potent antitumor and antiangiogenic effects in mouse tumor models
and in humans with certain malignancies.[2-4] Toceranib is currently approved for the treatment of mast cell tumors in dogs. The drug also appears to have biological activity against other
tumors in dogs such as mammary carcinoma, soft tissue sarcoma, and anal gland adenocarcinoma.[1, 5]
In addition to their cytotoxic effects on tumor cells, recent data suggest that TKIs may also have immunomodulatory effects, particularly on key immune cells recruited by tumors such as
myeloid-derived suppressor cells (MDSCs) and regulatory T-lymphocytes (Treg). These cell populations play central roles in immune suppression, and reversal of their effects is critically
important to the success of cancer immunotherapy. For example, in a mouse tumor model, treatment with sunitinib decreased MDSC accumulation, prevented Treg development, and improved the
efficacy of a tumor immunotherapy protocol.[6] In humans with renal cell carcinoma, sunitinib treatment appeared to reverse peripheral immune dysfunction, primarily by stimulation of a type-1
(Th1) T-cell-mediated immune response. Treg isolated from these patients demonstrated diminished suppressive function against autologous effector T cells.[7] Although the mechanisms for these
effects are not completely understood, sunitinib and other TKIs appear to modulate Treg indirectly, largely through suppressive effects on MDSCs and tumor-associated macrophages.[8]
Therefore, there is considerable interest in the immunomodulatory properties of TKIs and in their potential combination with cancer immunotherapy.
Because of their central role in tumor-induced immune suppression and correlation with a poorer prognosis in many human tumors, Treg represent an attractive therapeutic target. Although the
clinical relevance of Treg is not yet known in dogs with cancer, Treg are present in increased numbers in the blood and regional lymph nodes of dogs with various malignancies and may be
associated with outcome.[9, 10] Similar to sunitinib, the alkylating chemotherapy agent cyclophosphamide (CYC) also is known to selectively decrease Treg and inhibit their function in mice
and humans with cancer. In these species the effects of CYC on Treg occur through both direct and indirect effects of the drug.[11-13] We recently demonstrated that daily oral administration
of low-dose CYC to dogs with soft tissue sarcoma led to a selective decrease in the absolute number and percentage of Treg in peripheral blood, suggesting that CYC may have similar
immunomodulatory effects on canine Treg.[14]
Low-dose CYC and TKI treatment are attractive treatment approaches for companion animals with cancer. This is largely because of the ease of oral administration of these drugs compared to
conventional chemotherapy protocols. Combination treatment with low-dose CYC and toceranib has not been previously described, nor have the effects of toceranib on Treg and other T-lymphocyte
subsets been determined. Therefore, we designed a prospective study to evaluate the effects of toceranib, administered as a single agent and then in combination with low-dose CYC, on
circulating Treg numbers and percentages in dogs with various malignancies. In addition, we sought to determine the safety of combination toceranib and metronomic CYC treatment. We
hypothesized that administration of toceranib would selectively decrease Treg numbers in the blood of dogs with cancer and that the addition of CYC would further enhance this decrease. We
also expected the drug combination to be well tolerated in dogs with advanced disease.
Materials and Methods
Inclusion and Exclusion Criteria
Dogs presenting to the Colorado State University Veterinary Teaching Hospital with a histologically or cytologically confirmed diagnosis of any type of neoplasia except mast cell tumor
were eligible for inclusion in this prospective clinical trial provided that they had either failed standard of care treatment or their owners declined other treatment options. Although
c-Kit mutations are reported in other canine malignancies, mast cell neoplasms were excluded with the rationale that their relatively high mutation rate might lead to a variable
biologic response to toceranib between dogs with wild type or mutant tumors.[15, 16] Eligible dogs were required to have at least 1 measurable tumor, no clinically relevant biochemical or
hematological abnormalities, a performance status of 0 or 1, and a life expectancy of 8 weeks. Previous treatment was allowable with a 7-day washout period from surgery,
chemotherapy, or radiation treatment. Concomitant medications such as analgesics and those needed to manage toxicities also were permitted; no other drugs could be started during the
study period. This study was approved by the Animal Care and Use Committee at Colorado State University and signed informed consent was obtained from all owners before study entry.
Information Obtained
Information recorded included signalment, body weight, tumor type, clinical stage, measurement of at least 1 tumor lesion, toceranib and toceranib/cyclophosphamide toxicoses, concurrent
medications, clinical response, date of tumor progression, date of death and, when available, cause of death and necropsy results. Pretreatment evaluation included CBC, blood chemistry
panel, and urine analysis. Thoracic radiographs or abdominal ultrasound examination was also performed if needed to obtain tumor measurements.
Treatment Protocol and Sample Collection
After collection of a baseline peripheral blood sample for lymphocyte subset and cytokine analysis, dogs received toceranib (generously provided by Pfizer Animal Health) at a target
dosage of 2.75 mg/kg PO every other day for 14 days. Adverse events (AE) were managed when necessary by the institution of a drug holiday of up to 2 weeks followed by a
dose reduction to 2.75 mg/kg on a Monday-Wednesday-Friday schedule. For these dogs, day 0 was reset and 2 weeks of toceranib treatment was again required before CYC could be
administered. Oral CYC at a target dosage of 15 mg/m2/day then was added to toceranib starting at day 14 (or 2 weeks after toceranib had been given at a tolerable
dose). The dosage of CYC was chosen based on our recent investigation of the effects of CYC on Treg numbers in the blood of dogs with soft tissue sarcoma.[14] To improve the accuracy of
drug administration, CYC was compounded into 2.5 and 5 mg capsules.
A CBC and whole blood for collection of peripheral blood mononuclear cells (PBMCs) and serum were obtained at days 7, 14, 28, 42, and 56 after starting toceranib treatment. In addition a
chemistry panel, urine analysis, tumor measurements and thoracic or abdominal ultrasound examination or both (if necessary to determine tumor response) were obtained at days 28 and 56 of
the study. The longest diameter of the tumor (or tumors) was measured at each time point and response was assessed by Response Evaluation Criteria in Solid Tumors (RECIST).[17] Safety
assessments included AE, hematology, clinical chemistry profiles, and urinalyses based on the Veterinary Co-Operative Oncology Group Common Terminology Criteria for Adverse Events
(VCOG-CTCAE) v1.[18] Clients completed a dog quality of life questionnaire at each visit.
Sample Preparation
PBMCs were prepared within 30 minutes from blood samples collected in EDTA tubes. After lysis of red blood cells with an ammonium chloride buffer (ACK), cells were plated at a
concentration of approximately 1 × 106 cells per well in 96-well plates. PBMCs then were immunostained for surface expression of CD4 and CD8 with FITC-conjugated
anti-canine CD4 monoclonal antibody (clone YKIX302.9)2 and Alexa 647-conjugated anti-canine CD8 monoclonal antibody (clone YCATE55.9)3 and for B cells with PE-conjugated anti-canine CD21
monoclonal antibody (clone CA2.1D6)3 following the method described previously.[19] Immunostaining for FoxP3 expression was performed as previously described with a cross-reactive,
PE-conjugated murine FoxP3 antibody (clone FJK-16s).4,[20] A directly conjugated rat IgG2A antibody was used as the isotype control. Serum samples were stored frozen
at −80°C until analysis.
Flow Cytometric Analysis
Flow cytometry was performed with a CyAn ADP-flow cytometer5 and Flow Jo software6 for data analysis. Analysis gates were set on the live lymphocyte population based on typical forward
and side scatter characteristics.[21] Treg were identified as based on dual expression of CD4 and FoxP3 following the method described previously.[20] The percentage of Treg was
calculated by determining the percentage of CD4+FoxP3+ cells within the CD4+ T-cell population. Percentages of CD4+ and CD8+ T cells and B cells also were determined. Absolute numbers of
Treg, CD4+ and CD8+ T cells and B cells in peripheral blood were calculated based on the total lymphocyte count determined from a CBC performed with an automated cell counter.
In vitro Expansion of Treg
Canine lymphocytes were activated and cultured in IL-2 and TGF-β by means of a previously described protocol that expands a population of CD4+FoxP3+ T cells with phenotypic and functional
properties consistent with Treg.[20] Briefly, PBMC were obtained from 3 healthy dogs and cultured at 2 × 106/mL in media7 containing 10% fetal bovine serum and 1% each of
penicillin/streptomycin and L-glutamine in 24-well plates. The cells were stimulated with recombinant human IL-2 and TGF-ß8 and Concanavalin A9 (ConA) for 5 days at 37°C in 5%
CO2. After 4 days of culture, toceranib was added to some of the cultures at either 10 or 100 ng/mL. These concentrations were chosen based on the range achievable in the
serum of dogs receiving the drug.[22] Twenty-four hours later, the cells were harvested, stained with CD4-FITC and Foxp3-PE, and analyzed by flow cytometry as described above.
Cytokine Analysis
Serum samples were thawed and analyzed for canine IFN-γ by ELISA with a Duoset kit8 according to the manufacturer's directions. All patient samples were run in triplicate.
Statistical Analysis
Changes in mean percentages and absolute numbers of Treg between pretreatment and after 14 days of toceranib treatment were compared by paired, 1-tailed t-tests; hematologic
parameters, and mean percentages and absolute numbers of CD4+ and CD8+ T cells and B cells were compared for the same time points by paired, 2-tailed t-tests. All comparisons
between multiple time points were performed by means of a one-way ANOVA with Tukey's multiple means comparison. Correlation between changes in IFN-γ concentrations and Treg numbers was
determined by linear regression analysis. Statistical calculations were performed by means of a commercial software program.9 A P-value of < .05 was considered
significant for all analyses.
Results
Study Patients
A total of 7 male and 8 female dogs were enrolled in this prospective clinical trial. Two of the male dogs and 1 of the female dogs were intact and the remaining dogs were neutered. Breeds
included 2 Labrador Retrievers, 1 Rottweiler, 1 Boxer, 1 Saint Bernard, 1 Norwegian Elkhound and 1 Miniature Schnauzer, with the remainder being mixed-breed dogs. The median age was 10
(range, 2–13) years and median body weight was 28.9 (range, 11.3–51) kg. Nine of the 15 dogs received 1 or more medications for pain during the study period (including carprofen, tramadol,
gabapentin, amantadine or some combination of these) and 6 dogs received maropitant or metronidazole as needed to manage nausea or diarrhea, respectively.
Tumor types included osteosarcoma of the appendicular or axial skeleton in 3 dogs and 1 dog, respectively, soft tissue sarcoma in 4 dogs, and 1 each of hemangiosarcoma, lymphangiosarcoma,
malignant histiocytosis, transitional cell carcinoma (TCC) of the urethra, thyroid carcinoma, anal sac adenocarcinoma, maxillary carcinoma, and carcinosarcoma of the perineum. One dog had 2
separate tumors (a urethral TCC and a soft tissue sarcoma of the body wall). At the time of study initiation, 12 of the 15 dogs had metastatic disease to local lymph nodes, lung, liver,
spleen, bone, or some combination of sites. For the 13 dogs that were enrolled through at least the first 4 weeks of the 8-week study period, the mean toceranib dosage was 2.67 (range,
2.67–2.79) mg/kg and the mean CYC dosage was 15.46 (range, 13.71–16.62) mg/m2. Of these dogs, 6 had stable disease throughout the 8-week study period whereas 7 were determined to
have progressive disease at either the 6- or 8-week study visit. Two dogs were removed from the study before beginning CYC treatment because of rapidly progressive disease and therefore
received toceranib alone. All 15 dogs have either died or been euthanized because of clinical signs attributable to progression of their disease.
Combination of Low-Dose CYC with Toceranib Is Well Tolerated
We first investigated the safety of toceranib and metronomic dosing of oral CYC. This study was conducted by means of a standardized quality of life assessment form completed by the owner at each
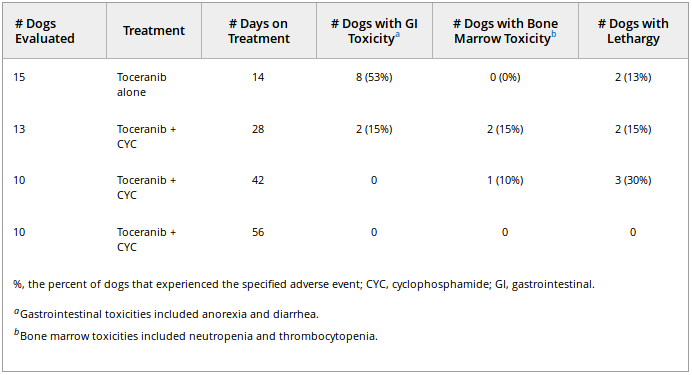
study visit and graded according to the VCOG-CTCAE v1.0. All toxicities were either grade 1 or 2 in nature and consisted of gastrointestinal (anorexia, diarrhea, or both), bone marrow
(neutropenia or thrombocytopenia) or lethargy (see Table 1). Gastrointestinal AE were most common, occurring most frequently during the first 2 weeks of the study in which toceranib was
the only drug administered. During this period, 33% (5 of 15) of dogs developed either grade 1 or 2 diarrhea whereas 20% (3/15) had either grade 1 or 2 anorexia, with 2 having both. For these 2
dogs, a drug holiday of 10–14 days was sufficient to manage gastrointestinal toxicity and toceranib then was reinstituted at 2.75 mg/kg on a Monday-Wednesday-Friday schedule. At day 28,
when CYC and toceranib had been given concurrently for 2 weeks, the incidence of gastrointestinal toxicity was 15%, with only 2 of 13 dogs experiencing either diarrhea or anorexia.
Table 1. Adverse events (either grade 1 or 2) for dogs receiving toceranib alone or toceranib
combined with low-dose cyclophosphamide.
Hematologic AE were uncommon with only 1 dog experiencing grade 1 neutropenia (at both day 28 and day 42), whereas another developed grade 1 thrombocytopenia after 2 weeks of combination
treatment (day 28). Neither dog required any changes to its treatment protocol. The dog with thrombocytopenia developed clinically relevant progression of splenic malignant histiocytosis within
1 week of becoming thrombocytopenic and was removed from the study. The persistent low-grade neutropenia in the other dog was resolved by day 56. Mild (grade 1) to moderate (grade 2)
lethargy was the only other toxicity reported; grade 2 lethargy reported in 2 dogs at the day 42 visit resolved with continuation of daily CYC treatment and reduction of toceranib to a
Monday-Wednesday-Friday schedule.
Toceranib Selectively Decreases Treg in the Blood of Dogs with Cancer
To determine the effects of toceranib on numbers of Treg in circulation, blood samples were collected on days 0 and 14 after beginning toceranib treatment and changes in the mean absolute number
and percentage of Treg were determined as described in the “'Materials and Methods'.” Both the percentage and absolute number of Treg in peripheral blood were significantly decreased after
toceranib treatment compared to pretreatment samples (Fig 1). The mean percentage of Treg before receiving toceranib was 9.6 (±5.8)% compared to 4.9 (±1.4)% after 14 days of toceranib
treatment (P = .004), while the mean absolute number of pretreatment Treg was 44.6 (±22.7 cells/μL) compared to 26.2 (±12.9 cells/μL) at day 14
(P = .001). To determine whether toceranib had an effect on other lymphocyte populations, numbers of CD4+ and CD8+ T cells and B cells were measured. Despite the
significant decrease in Treg, there were no changes in the absolute numbers or percentages of either CD4+ or CD8+ T cells or B cells during the 2-week period, suggesting that the effects of
toceranib were selective for the Treg subset of T-lymphocytes (see Table 2).
Figure 1: Relative and absolute numbers of regulatory T cells (Treg) in peripheral blood of dogs with cancer treated with toceranib at a target
dose of 2.75 mg/kg every other day (n = 15). The mean percentage of Treg (expressed as a percentage of the CD4 + T cell population) in blood before starting toceranib
and after 14 days of treatment were plotted in (A) and the mean of the absolute number of Treg at the same time points were plotted in (B). Error bars show SD.
Table 2. Relative and absolute numbers of lymphocyte subpopulations in 15 dogs with cancer
treated with toceranib for 14 days.
Treg Numbers Remain Low after Addition of Low-Dose CYC to Toceranib
We next examined whether dogs receiving oral CYC in addition to toceranib experienced further reductions in circulating Treg numbers. This was done by comparing Treg numbers in the blood of dogs
at the day 14 time point (before beginning CYC) with Treg numbers at each subsequent visit. Although there were significant differences between percentages of Treg at day 14, 42, and 56 compared
to baseline at day 0 (P = .002), there was no clear indication that the addition of CYC resulted in a further decline over that achieved with toceranib alone
(Fig 2A). Evaluation of absolute numbers of Treg in blood showed a similar response (Fig 2B) with significantly fewer Treg at day 14 versus day 0 and at day 56 versus day 0
(P = .004) but not between day 14 and later time points.
Figure 2: Relative and absolute numbers of Treg in peripheral blood of dogs with cancer receiving toceranib combined with metronomic
cyclophosphamide (CYC). Dogs started toceranib at day 0 and then CYC was added at a target dose of 15 mg/m2/day at day 14. Blood samples were collected for Treg analysis at days
0, 14, 28, 42, and 56 relative to beginning toceranib. The mean percentage of Treg (±SD) was plotted in (A) and the mean of the absolute number (±SD) of Treg was plotted in (B). Asterisk (*)
denotes significant change from day 0.
Toceranib's Effects on Treg May Be Indirect
The preceding data suggested that administration of toceranib was associated with a decline in Treg in the blood of dogs with cancer. Given this negative relationship, we tested whether or not
toceranib directly affected the expansion of Treg in vitro. This question was addressed by adding toceranib to cultures of Treg that had been previously expanded for 4 days with ConA plus
IL-2 and TGF-β (see “'Materials and Methods'”). The percentage of Treg in culture then was determined by flow cytometry. As shown in Figure 3, neither concentration of toceranib inhibited
the expansion of Treg compared to control conditions during the 24-hour coincubation period. Therefore, it is possible that the negative effect toceranib has on Treg in vivo is not a direct
result of the drug on Treg outgrowth or viability, as has been suggested in similar experiments with human cells using the TKI sunitinib.[7]
Figure 3: In vitro expansion of Treg with or without the addition of toceranib. Peripheral blood mononuclear cells (PBMC) were collected from
normal dogs (n = 3) and placed in culture in to expand the Treg population. After 4 days, toceranib at either 10 or 100 ng/mL was added for the last 24 hours. The mean
percentages of Treg between the culture conditions are plotted. Error bars show SD.
Decreases in Treg Correlate with Increased Concentrations of IFN-γ in Dogs Receiving Toceranib and Low-Dose CYC
Because related TKIs such as sunitinib have been shown to restore IFN-γ-mediated T cell responses as Treg numbers decrease in human cancer patients, we next investigated whether or not the
decrease in circulating Treg observed in dogs in this study was associated with an increase in the serum concentration of IFN-γ.[23, 24] To accomplish this we measured the concentration of this
cytokine in the serum of study dogs by ELISA at each time point after administration of toceranib or combination toceranib/low-dose CYC. The mean concentration of IFN-γ was significantly higher
than baseline for all time points in which dogs had been receiving both CYC and toceranib (Fig 4A). Interestingly, a significant inverse correlation was found between the increase in serum
IFN-γ concentration and the decrease in Treg numbers after 6 weeks of combination treatment (Fig 4B) (r2 = 0.54, P < .02) but not at
earlier time points. This suggests that either prolonged administration of toceranib or toceranib combined with CYC might be necessary to increase IFN-γ concentrations.
Figure 4: Interferon gamma (IFN-γ) production and correlation with changes in circulating Treg numbers in dogs receiving toceranib and
cyclophosphamide (CYC). The mean serum concentration of IFN-γ for each time point is plotted in (A). Asterisk (*) denotes significant change from day 0. In (B) the correlation between the log
change in IFN-γ concentration and log change in Treg number at day 0 and day 56 is plotted.
Discussion
Several important findings emerged from these studies. First, we found that administration of toceranib was associated with a significant and selective decrease in the number and percentage
of Treg in the blood of dogs with cancer. When a metronomic dosage of CYC was combined with toceranib, Treg numbers remained low although serum concentrations of the proinflammatory cytokine
IFNγ increased significantly, suggesting potential amelioration of the immunosuppressive effects of Treg. In addition, the combination of toceranib and low-dose CYC was well tolerated in dogs
with advanced neoplasia. Although tumor efficacy was not a study endpoint, 6 of 15 dogs had stable disease for the 8-week study period. Therefore, toceranib combined with metronomic CYC may
represent an attractive treatment option for dogs with cancer based on potential antitumor and immunomodulatory effects.
Toceranib has demonstrated therapeutic activity in dogs with mast cell tumors and several other tumor types. The data presented herein indicate that decreasing numbers of circulating Treg may
be another mechanism of action of toceranib. Although preliminary, our in vitro data suggest that Treg modulation may occur through indirect rather than direct effects of the drug because,
under conditions known to expand the Treg subset from activated lymphocytes, we were unable to detect a difference in Treg percentages between cultures that contained toceranib and those that
did not. This result is consistent with those of studies in human cancer patients in which clinically relevant doses of sunitinib decreased peripheral Treg but had no effect on the ability of
either Treg or other T effector cells to expand in vitro despite periods of drug exposure as long as 14 days.[7] Unfortunately, drug exposure times longer than 24 hours were not assessed in our study; therefore more delayed effects on Treg viability cannot be
ruled out. In addition, this study was limited by the use of 2 markers (CD4 and FoxP3) to identify the canine Treg population; inclusion of the recently developed canine-specific anti-CD25
antibody as a third marker might have increased the accuracy of Treg identification.[25, 26] Because some dogs received other medications
during the course of this clinical trial, we also cannot exclude the possibility that other drugs might also impact Treg numbers or interact with toceranib's effects on this T cell subset.
This might be particularly relevant to the 4 dogs that received carprofen during the study period, because data from mouse tumor models suggest that inhibition of cyclooxygenase-2 also may
suppress Treg function.[27]
We recently completed a pilot study evaluating the effects of low-dose CYC on circulating Treg numbers in dogs with soft tissue sarcoma.[14] In that study, we found that CYC administered orally at dosages of either 12.5 or 15 mg/m2/day resulted in a significant decrease in
the number of circulating Treg within a 28-day period of time. Therefore, we expected that the addition of CYC at 15 mg/m2/day to toceranib treatment would elicit a further
decrease in circulating Treg numbers compared to that achieved with toceranib alone. This notion also is consistent with data from preclinical studies that demonstrate the cooperative
anti-angiogenic effects of TKIs and CYC.[28, 29] However, although Treg were significantly
decreased after 6 weeks of combination treatment compared to pre-study values (Fig 2), they were not different from day 14 when only toceranib had been administered. Although it is possible that combination treatment was required to achieve the
low value at day 56, it could also be true that prolonged toceranib treatment alone might result in a similar number. Indeed, a weakness of this study was the absence of a group of dogs that
received single agent toceranib treatment over the same 8-week period. Such a group might have been helpful in assessing chronic drug effects on Treg numbers. Other factors, such as drug
interactions and the effects of tumor type or stage on Treg, also may have obscured detection of any synergistic activities of the 2 drugs.
Decreases in peripheral Treg numbers frequently are associated with improved cell-mediated immune responses in humans with cancer and in mouse tumor models. In many clinical trials of humans,
these responses are monitored with concentrations of key Th1 cytokines such as IFN-γ. Therefore, we hypothesized that the decrease in Treg numbers observed in the dogs of this study would
correspond to an increase in serum concentrations of IFN-γ. Unexpectedly, despite the sharp decrease in Treg numbers, the concentration of IFN-γ after 2 weeks of toceranib treatment was
similar to pretreatment concentrations. In fact, a significant increase from baseline concentrations was not observed until 2 weeks after toceranib and CYC were being given concurrently.
Interestingly, the inverse relationship between the increased concentration of IFN-γ and decreased numbers of Treg was significant only after combination treatment had been given for
6 weeks, at the time when Treg numbers were at their lowest. One possible explanation for these results is that sufficiently low numbers of Treg are required before suppressed Th1 immune
responses can recover. Given that CYC has been shown to promote the secretion of IFN-γ from CD8+ T cells, it is also possible that CYC improves nonregulatory T cell function in dogs by
stimulating secretion of IFN-γ from Th1 T cells or through activation of other cells such as dendritic cells.[30, 31] Mechanistic studies examining the effects of both drugs on the function of Treg and other T cell subsets in dogs will be required to gain a
better understanding of their immunomodulatory properties.
Toxicity of combined CYC and toceranib was characterized as being mild (grade 1) or moderate (grade 2) in all the dogs of this study. The majority of gastrointestinal AE occurred during the
first 2 weeks, at a time when only toceranib was being administered. This is consistent with the results of other studies with toceranib in which gastrointestinal toxicities
predominate.[32] Only 1 dog developed mild neutropenia during our study. Although combination of toceranib with other drugs such as vinblastine has been limited
by neutropenia, the results of our previous work with CYC at 15 mg/m2/day suggested that bone marrow toxicity from combination treatment was unlikely. In other studies
evaluating metronomic dosing of CYC, sterile hemorrhagic cystitis (SHC) has been reported in as many as 10–22% of dogs.[33, 34] Although the duration of CYC administration in our study was short (6 weeks), clinical and laboratory signs consistent with SHC were
not observed.
In conclusion, we found that the combination of toceranib and low-dose CYC was safe in dogs with cancer. In addition, administration of toceranib resulted in a selective decrease in the
number and percentage of Treg in the peripheral blood. Our data suggest that the addition of CYC may further modulate canine Treg, or potentially other immune cells, by stimulating secretion
of IFN-γ. Because of the small number of dogs in this study, these results must be considered preliminary, but they support additional studies to better characterize the antitumor and
immunomodulatory effects of combination toceranib and low-dose CYC treatment.
Acknowledgments
The authors thank the oncology clinical trials service at the Animal Cancer Center for their excellent support of this project.
This study was supported by a grant from the College Research Council at Colorado State University.
Footnotes
-
1
Palladia, Pfizer Animal Health, New York, NY
-
2
Serotec, Raleigh, NC
-
3
Mouse FoxP3 staining kit, eBioscience, San Diego, CA
-
4
Cyan ADP-flow cytometer, Dako-Cytomation, Fort Collins, CO
-
5
Flow Jo software, v.7.2.5, Flow Jo, Ashland, OR
-
6
RPMI, Invitrogen, Carlsbad CA
-
7
Peprotech Rocky Hill, NJ
-
ConA, Sigma Aldrich, St. Louis, MO
-
8
IFN-γ and VEGF-α ELISA kits, R&D Systems, Minneapolis, MN
-
9
GraphPad Prism, version 5 for Windows, GraphPad Software, San Diego, CA
References
-
1London CA, Hannah AL, Zadovoskaya R, et al. Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with
spontaneous malignancies. Clin Cancer Res 2003;9:2755–2768.
-
2Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther 2005;315:971–979.
-
3Shawver LK, Slamon D, Ullrich A. Smart drugs: Tyrosine kinase inhibitors in cancer therapy. Cancer Cell 2002;1:117–123.
-
4Hynes NE, Lane HA. ERBB receptors and cancer: The complexity of targeted inhibitors. Nat Rev Cancer 2005;5:341–354.
-
5London CA, Malpas PB, Wood-Follis SL, et al. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a
receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin Cancer Res 2009;15:3856–3865.
-
6Ozao-Choy J, Ma G, Kao J, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor
microenvironment for immune-based cancer therapies. Cancer Res 2009;69:2514–2522.
-
7Finke JH, Rini B, Ireland J, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma
patients. Clin Cancer Res 2008;14:6674–6682.
-
8Yang Z, Zhang B, Li
D, et al. Mast cells mobilize myeloid-derived suppressor cells and Treg cells in tumor microenvironment via IL-17 pathway in murine
hepatocarcinoma model. PLoS ONE 2010;5:e8922.
-
9Biller BJ, Guth A, Burton JH, et al. Decreased ratio of CD8 + T cells to regulatory T cells associated with decreased survival in dogs with
osteosarcoma. J Vet Intern Med 2010;24:1118–1123.
-
10Horiuchi Y, Tominaga M, Ichikawa M, et al. Relationship between regulatory and type 1 T cells in dogs with oral malignant melanoma. Microbiol Immunol 2002;54:152–159.
-
11Zou W. Regulatory T cells, tumour immunity
and immunotherapy. Nat Rev Immunol 2006;6:295–307.
-
12Matsushita N, Pilon-Thomas SA,
Martin LM, et al. Comparative methodologies of regulatory T cell depletion in a murine melanoma model. J Immunol Methods 2008;333:167–179.
-
13Langroudi L, Hassan ZM, Ebtekar M, et al. A comparison of low-dose cyclophosphamide treatment with artemisinin treatment in reducing the number of regulatory T
cells in murine breast cancer model. Int Immunopharmacol 2010;10:1055–1061.
-
14Burton JH, Mitchell L, Thamm DH, et al. Low-dose cyclophosphamide selectively decreases regulatory T cells and inhibits angiogenesis in dogs with soft tissue
sarcoma. J Vet Intern Med 2011;25:920–926.
-
15Gregory-Bryson E, Bartlett E,
Kiupel M, et al. Canine and human gastrointestinal stromal tumors display similar mutations in c-KIT exon 11.
BMC Cancer 2010;10:559.
-
16Usher SG, Radford AD, Villiers EJ, et al. RAS, FLT3, and C-KIT mutations in immunophenotyped canine leukemias. Exp
Hematol 2009;37:65–77.
-
17Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and
Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–216.
-
18Veterinary Co-operative Oncology Group. Common Terminology Criteria for Adverse Events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.0. Vet Comp Oncol 2004;2:195–213.
-
19Walter CU, Biller BJ, Lana SE, et al. Effects of chemotherapy on immune responses in dogs with cancer. J Vet Intern
Med 2006;20:342–347.
-
20Biller BJ, Elmslie RE, Burnett RC, et al. Use of FoxP3 expression to identify regulatory T cells in healthy dogs and dogs with cancer. Vet Immunol Immunopathol 2007;116:69–78.
-
21Faldyna M, Leva L, Knotigova P, et al. Lymphocyte subsets in peripheral blood of dogs—a flow cytometric study. Vet
Immunol Immunopathol 2001;82:23–37.
-
22Yancey MF, Merritt DA, White JA, et al. Distribution, metabolism, and excretion of toceranib phosphate (Palladia, SU11654), a novel tyrosine kinase inhibitor,
in dogs. J Vet Pharmacol Ther 2010;33:154–161.
-
23Motzer RJ, Hoosen S, Bello CL, et al. Sunitinib malate for the treatment of solid tumours: A review of current clinical data. Expert Opin Investig Drugs 2006;15:553–561.
-
24Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA
2006;295:2516–2524.
-
25Abrams VK, Hwang B, Lesnikova M, et al. A novel monoclonal antibody specific for canine CD25 (P4A10): Selection and evaluation of canine Tregs.
Vet Immunol Immunopathol 2010;135:257–265.
-
26Pinheiro D, Singh Y, Grant CR, et al. Phenotypic and functional characterization of a CD4(+) CD25(high) FOXP3(high) regulatory T-cell population in the
dog. Immunology 2011;132:111–122.
-
27Sharma S, Yang SC, Zhu L, et al. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4 + CD25 + T
regulatory cell activities in lung cancer. Cancer Res 2005;65:5211–5220.
-
28Blansfield JA, Caragacianu D,
Alexander HR 3rd, et al. Combining agents that target the tumor microenvironment improves the efficacy of anticancer
therapy. Clin Cancer Res 2008;14:270–280.
-
29Zhang L, Smith KM, Chong AL, et al. In vivo antitumor and antimetastatic activity of sunitinib in preclinical neuroblastoma mouse model.
Neoplasia 2009;11:426–435.
-
30Ghiringhelli F, Menard C, Terme M, et al. CD4+ CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent
manner. J Exp Med 2005;202:1075–1085.
-
31Schiavoni G, Sistigu A, Valentini M, et al. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of
immunogenic tumor apoptosis. Cancer Res 2011;71:768–778.
-
32London CA. Tyrosine kinase inhibitors in
veterinary medicine. Top Companion Anim Med 2009;24:106–112.
-
33Lana S, U'Ren L, Plaza S, et al. Continuous low-dose oral chemotherapy for adjuvant therapy of splenic hemangiosarcoma in dogs. J Vet Intern Med 2007;21:764–769.
-
34Elmslie RE, Glawe P, Dow SW. Metronomic therapy with cyclophosphamide and piroxicam effectively delays tumor recurrence in dogs with incompletely resected soft
tissue sarcomas. J Vet Intern Med 2008;22:1373–1379.
Share this article / Teilen Sie diesen Artikel