
Article added / Artikel hinzugefügt 01.10.2021
Generally Articles and Discussions about Osteosarcoma in Dogs
→ Evaluations of phylogenetic proximity in a group of 67 dogs with
osteosarcoma: a pilot study
Article added / Artikel hinzugefügt 01.10.2021
Generally Articles and Discussions about Osteosarcoma in Dogs
→ Canine Periosteal Osteosarcoma
Images added / Abbildungen hinzugefügt 02.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Pulmonary vessels
New subcategory added / Neue Unterkategorie hinzugefügt 02.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Pulmonary vessels
Images added / Abbildungen hinzugefügt 01.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Heart valvular diseases



Canine Osteosarcoma: A Review and an Experimental Treatment Regime

Johnson, Philip; Runyon, C.; and Grier, R. L. (1981) "Canine Osteosarcoma: A Review and an Experimental Treatment Regime," Iowa State University Veterinarian: Vol. 43: Iss. 1, Article 4. Available
at: http://lib.dr.iastate.edu/iowastate_veterinarian/vol43/iss1/4

Summary
Osteosarcoma, the major form of bone
cancer in dogs, is reviewed. Incidence rates
relative to breed, age, and sex characteristics
are outlined. Prediliction sites are also stated.
The clinical, radiographic, and metasta-
tic characteristics of osteosarcoma are ex-
plained.
Theories on etiology including work by
Brodey, Wolke and Nielson are discussed.
Alternate treatment regiments are examined,
including an indepth look at a case at the
Iowa State University Clinic which was
treated using a combination of ostectomy and
allograft, local hyperthermia, bleomycin, and
levamisole.
A Review of Osteosarcoma
Osteosarcoma is the major form of bone
cancer seen in the dog. 6,10,16,22,30 One study
showed that 85% of the primary bone tumors
seen in dogs. were osteosarcoma, while
chondrosarcomas, the second most common
primary bone tumor, occurred only 10% of
the time.6
Canine osteosarcoma is nearly
always malignant, as compared to a 50%
malignancy rate in cats and a generally
benign situation in cattle and in horses. 22
Several surveys have shown that large and
giant breeds have a much higher incidence of
osteosarcoma and are at a significantly
greater risk of devleoping osteosarcoma than
smaller breeds. 5,6,10,19,29,30 Among giant dogs
the risk of bone sarcoma is estimated to be 5
to 30 times the risk of any other cancer. The
excess risk of bone sarcoma appears to be
characteristic of large breeds as a group and
not of one or several particular breeds.29
The incidence of osteosarcoma increases
in middle aged and older dogs.6,7,10,19 Giant
dogs with osteosarcoma seem to be slightly
younger at the time of disease development
than those in other weight groups.19 The
average age in a survey of 194 osteosarcoma
cases was 7.7 years 6, while in another survey
of 65 cases the median age was 6.0 years.5
Most surveys of osteosarcoma indicate that
the incidence is higher in males than
females5,6,19 but at least one survey failed to
note a difference in incidence rate between
the sexes.10 The surveys stating a higher male
to female ratio varied slightly in their
numbers with ratios of 1.2:1.06 ,3.0:2.05, and
1.7:1.019 being reported.
Most cases of osteosarcoma occur in the
appendicular skeleton, primarily in the long
bones.6,7,16,17,30 There was a higher incidence
in the pectoral limbs than in the pelvic
limbs.6,30 A study of Wolke and Nielson
showed 47% of the total cases of osteosar-
coma occurring in the pectoral limbs, while
29% of the cases occurred in the pelvic limb.
This figures out to a 1.6: 1.0 ratio,
corresponding to the ratio of weight
distribution between front and rear legs.3o
Six sites in the long bones have the highest
incidence of osteosarcoma development.
These sites are the proximal humerus, distal
radius, proximal and distal femur, and prox-
imal and distal tib~a.6,7,I6,I7,30In the Wolke
and Nielson survey, the distal metaphysis of
the radius was the most common site, with
23% of the total cases. The proximal
metaphysis of the humerus was second in in-
cidence, with 19% of the cases.30
In some circumstances osteosarcoma may
originate in tissues other than bone. It has
been reported in the esophagus of a dog, ad-
jacent to a chronic lesion produced by the
spirurid worm, Spirocerca lupi. A second
extra-osseous site is in a mixed tumor of the
mammary gland. 26
One of the first clinical signs of osteosar-
coma in the metaphyseal region of a long
bqne is lameness. One to two weeks later there
is generally a cool, palpable swelling in th
area of the lesion. Eventually there is a visible
enlargement at the site of the lesion that is
warm and painful due to stretching of the
periosteum. 22
Radiographically, this tumor is usually
found at the extremity of a long bone and
produces a radiolucent enlargement arising in
the metaphysis which erodes the pre-existing
calcified bone of the cortex.26
The destructive process may be restricted to the medulla, but usually involves the cortex as well, by the time the tumor is manifested clinically. 27
In addition to cortical destruction,
another type of radiographic change that oc-
curs with osteosarcoma is periosteal response.
The degree of periosteal reaction does not de-
pend on the degree of cortical destruction.I7
This periosteal response can lead to a large
soft tissue mass contiguous to the bone. This
soft tissue swelling around the osteosarcoma
lesion is also related to reactive fibroplasia in
the subcutaneous and intramuscular tissues,
which leads to impaired circulation and
edema.27
All osteosarcomas are collagenoblastic
tumors in which the collagen fibers are
organized into varying amounts of osteoid,
bone, and cartilage. 22 Depending on which of
these components is dominant, three major
subtypes are recognized: osteoblastic,
fibroblastic, and chondroblastic. 12
The critical, identifying characteristic of
cells of osteosarcQma is their ability to pro-
duce ostedid.26
Osteoid is the collagenous matrix of bone, the primary product of the metabolic
activity of osteoblasts, which
possesses the specific bOinding sites of bone
mineral. 11
In primary bone neoplasms, when the
neoplastic bone cells have retained the ability
to produce osteoid, it is laid down in grossly
anomalous patterns. Mineralization takes
place as long as there is blood supply and the
retention ·of the basic molecular character-
istics of new collagen. A characteristic feature
of neoplastic bone is the inconsistency or
nonuniformiiy of the osteoid, reflecting the
degree of undifferentiation of the cells that
form iLII
As the tumor grows by this process of lay-
ing down osteoid, bone, and/or cartilage, the
periosteum in tne area of tumor growth can
be elevated. This elevation causes a triangle
to be formed where it joins normal cortex,
known as Codman's triangle. 17 This is
another distinctive radiographic feature of
osteosarcoma and is a valuable aid to
diagnosis.
Osteosarcoma does not often invade adja-
cent bone (i.e. in distal end of radius or tibia),
but this has been reported.23
More often, the adjacent bones may show radiographic evidence of periosteal reaction to the tumor. 17
This reaction causes new bone to be laid down
and gives the bone a rough appearance, sug-
gesting involvement with the tumor.
The metastatic route of osteosarcoma is
typically hematogenous.21,23 The lungs are
the'most common site of metastasis.6,17,23,27
Other sites of metastases are the liver,
kidneys, amputation stump6 and, on rare oc-
casion, to adjacent bones.
Neoplastic cells may embolize from the
site of origin without unusual trauma.
Manipulative trauma definitely increases the
number of cancer cells in circulating blood.
Both surgical and non-surgical trauma pro-
bably play a role in disseminating these cells
into the circulating blood. It has been sug-
gested that biopsy of malignant tumors of the
extemities should be performed under tourni-
quet whenever possible, and when indicated,
definitive, ablative opertions should be car-
ried out without releasing the tourniquet.21
The etiology of osteosarcoma is unknown,
but there have been several theories put for-
ward, all supported by at least some clinical
evidence. Brodey advances the theory that the
occurance of osteosarcoma can be correlated
with the high growth potentials of various
metaphyses of bones.6
For example, the distal
radius has a much higher growth potential
than the proximal radius and also has the
higher incidence of osteosarcoma of the two.
A similar situation exists with the proximal
humerus, which exceeds both the growth
potential and osteosarcoma incidence of the
distal humerus. Brodey continues with the
correlation by showing that the proximal and
distal femur and the proximal and distal tibia
have nearly equal growth potentials and a
nearly equal incidence of osteosarcoma.
Brodey hypothesizes this rapid, maximal
growth at the metaphysis in giant breed dogs
leaves behind small foci of retained hyaline
cartilage. These foci have not been seen in
smaller dogs. These foci may serve as sites of
origin for later tumor growth. 6
Wolke and Nielson consider other factors
to be involved in the etiology. They suggest
that weight bearing stresses on the metaphysis
of the long bones lead to the development of
osteosarcoma. They suggest that the re
higher incidence in the pectoral limbs versus
the pelvic limbs is directly proportional to the
relative weight distribution between the front
and back legs. They also site the increased in-
cidence in heavier dogs as further proof of
their weight-bearing stress theory.30 Another
study basically agrees with this theory, stating
that repeated trauma to the growth plates in
young giant breed dogs (caused by weight
bearing stresses), may partly be responsible
for the development of osteosarcomas at these
sites in later life. 14
Another theory concerns the relationship
of healed fractures to the development of
osteosarcoma. Bennett, Campbell and Brown
suggest that cartilage cells produced during
the healing of a fracture may persist long
after the fracture is healed, potentially form-
ing a focus for neoplastic development. 4 This
is similar to the Brodey theory of retained
hyaline cartilage cells providing the foci for
tumor growth, differing only in the origin of
the cartilaginous cells.
There have been reports of dogs and cats
that have developed tumors after metallic
surgical implants were used to treat bone
fractures. 2
,25 Implanted metals may form cor-
rosive products such as metallic salts or fine
particles. The animal's response to metallic
implants can vary from inflammation to
allergic reaction to tumorogenesis.14
A study of S clinical cases strongly supported
this theory. AIlS cases of osteosarcoma arose mid-
shaft of a long bone, a very atypical location,
and were in close proximity to a corroded
metallic implant. 25 Obviously, not every dog
that develops osteosarcoma has had a frac-
tured bone and/or a metallic implant, so
these last two theories are not the definitive
answer to the etiology of osteosarcoma, but
they may eventually help to find that answer.
Successful treatment of osteosarcoma has
advanced about as much as the search for its
cause. Amputation, irradiation therapy,
chemotherapy, immunotherapy and a com-
bination of these and other modalities have
been attempted with little success thus far. 5, 15
It is felt that early diagnosis is the key to the
success of any attempted therapeutic
regime. 17 Unfortunately this presents a very
early stumbling block in the battle against the
disease. By the nature of the disease, the
tumor may already be metastasized before it
is clinically recognized. In addition, clinical
recognition is often slow due to such things as
blaming early lameness on other minor trau-
matic episodes, radiographs not being taken
or poorly interpreted, or possibly even an in-
adequate biopsy being taken, missing the
diagnostic area of the lesion. 17
To overcome these problems of diagnosis,
all dogs with lamenesses- involving high in-
cidence sites, particularly in large or giant
breeds greater than two years old, should be
thoroughly examined. Radiographs should be
taken of the leg and carefully evaluated. If a
biopsy is to be performed, it should be done
with the aid of two radiographic views of the
suspected area. Broad areas of dense bone
should be avoided and a punch biopsy or a 2-
3mm thi.ck slice of tissue should be taken. The
cortex should be completely penetrated and
the medullary cavity entered. Post-operative
radiographs should be taken to evaluate the
success of the procedure. 17
The thorax should also be radiographed
when a malignant bone tumor is suspected. If
metastasis to the lungs has already occurred,
amputation is merely palliative and probably
should not not be done. Radiotherapy can be
used in these cases to ease pain and to slow
tumor growth. 5 Radiotherapy has been shown
to be of little benefit in other phases of
osteosarcoma treatment.It has failed to
resolve the primary tumor, to prevent
pulmonary metastasis and has undesirable
side effects on normal tisuses. Radiotherapy
may have also caused an increase incidence of
side effects from cytotoxic drugs in combina-
tion therapy. 15
Amputation of the diseased limb has been
the treatment of choice for several years, but
even with amputation the survival rate is
poor. Brodey points out that there is no
baseline data for long-term survival of dogs
with osteosarcoma that were not treated.
There are known cases where dogs with
osteosarcoma did survive without treatment,
and it is therefore concluded that not all long-
time survival can be credited to the treatment
under consideration, as some of those dogs
may have lived anyway.5
Chemotherapy and immunostimulants
have been recent developments in the fight
against osteosarcoma. Methotrexate, vincris-
tine sulfate, doxorubicin, cyclophosphamide,
and bleomycin are some of the many different
chemotherapeutic agents that have been or
are being tested. Thus far there is insufficient
data to determine if these drugs will be useful
or not.
The same observation is true for im-
munomodulators such as BCG (bacillus
Calmette-Guerin) vaccine and levamisole.
There is some evidence that BCG vaccine will
help delay metastasis following amputation
by activating macrophages non-specifically
and causing them to recognize and destroy
malignant cells. 15.
2o However, it has also been
demonstrated that BCG vaccine treatment, at
best, only delays and does not cure osteosar-
coma. More specific immunotherapy needs to
be developed.
Thus, even with therapy, the prognosis for
a dog with osteosarcoma is very poor. In one
study of 194 cases of osteosarcoma 85 %
were dead by 8 months, and of the other 15%, only
one dog was considered cured. 6
In another survey of 65 cases the results were
similar, with only 10.7% of the cases surviving one
year past the time of diagnosis. 5 There is some
hope that combination therapy and earlier
diagnosis will help to improve these figures.
Case Report-No. 582704-
An Experimental Treatment Regime
On June 24, 1980 a 7 year old, 80 pound,
mixed (Collie-Shepherd) spayed female dog
was presented to the Iowa State University
Small Animal Hospital with a history of
lameness in the left front leg of two days dura-
tion. The dog had been in a kennel for 10
days and had not been lame prior to board-
ing. At the time of admission there was
palpable swelling of the distal left radius.
Radiographs strongly suggested a primary
bone tumor such as osteosarcoma. At this
time the lungs showed no evidence of
metastasis.
A bone biopsy was taken and frozen sec-
tions indicated osteogenic sarcoma. Paraffin
sections confirmed this diagnosis. The owner
emphasized that he did not want the left
forelimb amputated and it was decided that
the dog would be released to return to the
clinic once a treatment protocol was
developed.
The dog returned to the clinic on July 14,
1980 and the left radius was again
radiographed. It was originally hoped to
debulk the tumor since at the time of first
presentation it had not invaded the opposite
cortex. However, the second set of
radiographs revealed rapid progression of the
osteosarcoma in the radius and marked in-
volvement of the opposite cortex. There was
still no evidence of pulmonary metastasis.
The soft tissue swelling was not very extensive.
Clinical pathology showed an elevated
alkaline phosphatase of 105.9 lUll (normal
10-80 lUll) which suggested bone cell activity
probably related to the osteogenic sarcoma.

The protocol for treatment was agreed
upon (Table 1). The regimen called for local
excision of the tumor, bleomycin chemother-
apy, levamisole immunomodulation and local
hyperthermia.

The distal one third to one half of the
radius, including the distal epiphysis and ar-
ticular surface, were removed. Frozen section
histopathology revealed that the proximal
end of the excised bone segment was free of
the tumor. Two screws were placed through
the proximal radius into the ulna to tem-
porarily stabilize the elbow joint. Two
Hemovac tubes were inserted in the incision
site for local hyperthermia treatment. The
perforated portion of each tube was placed in
the defect where the distal radius had
previously been. The rationale for adjunctive
local hyperthermia was the possibility of
tumor extension into soft tissues and proximal
to the excised portion of the bone as well as
the fragmentation of the neoplasm that oc-
curred at the time of excision.
The dog was given one half bolus, 92mg
or approximately 2.71 mg/kg, of levamisole 3
hours prior to surgery. Hyperthermia by
hydrothermic perfusion followed closure of
the wound, synchronized with 10 units of in-
travenous bleomycin. The log for the hyper-
thermia treatment can be found in Table 2.
In the course of the procedure the ther-
mometer was postitioned too close to the skin
on the far side of the leg. The tissue
temperature was thought to be too low during
the first part of the procedure, when actually
it was probably too high. Consequently the
tissue readings were in error and the possibili-
ty of thermal burn to the leg was high.
A lymphocyte transformation test was run
upon admission to the hospital on July 14,
1980. This test was used to measure the im-
mune status of the dog, and it indicated she
was immunosuppressed. (Table 4) This result
was not surprising due to the presence of a
well established neoplastic condition.
The dog was sent home 3 days post-
operatively with a surprisingly small area of
thermal burn.
The dog was re-admitted one week post-
operatively for the second phase of treatment.
The proximal incision was draining at this
time, and Pseudomonas was cultured from
the wound. The dog was put on Tribrissena
therapy for 5 days. The lymphocyte transfor-
mation test showed improvement of the im-
mune status. (Table 4) The dog was then
given her second hyperthermia treatment in
synchrony with 10 units of intravenous
bleomycin. At the end of the procedure the
tubes were removed. The log for the second
hyperthermia treatment can be found on
Table 3. The area of the thermal burn on the
proximal part of the leg was extensive after
the second hyperthermia treatment. The
burn was treated topically with sulfamylon
and bandages. The dog was sent home for the
weekend two days post-operatively.



The dog was re-admitted the following
Monday, July 28, 1980. The left leg was
radiographed and the ulna had fractured at
the distal screw due to excessive activity while
home. Bleomycin and levamisole treatments
were continued as called for by the protocol.
The wound cultured negative for Pseudo-
monas on two cultures, two days apart, so
systemic antibiotics were discontinued and
the burn was treated topically. The lym-
phocyte transformation test showed some
deterioration in the immune status of the dog.
A urinalysis and CBC were normal and the
alkaline phosphatase level was still elevated
with a 207 lUll. The dog was again sent
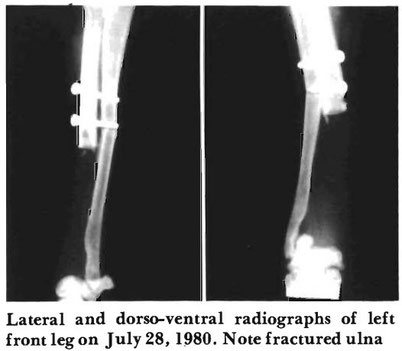
home with the leg encased in a Robert-Jones
dressing for protection and support.
On August 1, 1980 a whole cortical bone
allograft from a St. Bernard cross donor dog
was used to replace the distal one third to one
half of the radius. The radiocarpal joint was
arthrodesed and- both ends of the graft were
stabilized with Dynamic Compression Plates.
A cancellous bone graft from the greater
tubercle of the humerus was packed at the
ends and around the allograph. The
alignment was considered excellent on post·
operative radiographs. The thermal burns on
the lateral side of the leg were debrided and
sutured as much as possible.

The dog was placed on Ecotrinb for a few
days post-operatively to decrease discomfort.
On August 4, 1980 the wound again
cultured positive for Pseudomonas with only a
slight sensitivity to gentocin. Beginning
August 5, 1980 the dog was treated daily on
an out· patient basis. By August 12, 1980 the
wound over the proximal entry site of the
infusion tube was starting to granulate in and
the dog was showing steady improvement.
Lymphocyte transformation tests continued
to demonstrate immunosuppression. (Table
4) The bleomycin and levamisole therapy was
continued as ordered in the protocol.
The lymphocyte transformation test of
August 26, 1980 revealed the continued
immunosuppression. (Table 4) The dog is
bearing nearly full weight on the leg and the
wounds have nearly closed, but due to licking
by the dog, constant bandaging and con-
tinued Pseudomonas infection, the healing
process is slower than normal.
The lymphocyte transformation test was
repeated on September29, 1980 and again on
November 18, 1980. These tests continued to
show immunosuppression. (Table 4)
The most recent radiographs show the
ulna fracture to be healed and the allograft in
proper position and apparently becoming
incorporated into the bone as of December 5,
1980. There is no evidence of recurrence of
osteosarcoma at the primary, distal radial site
and the lungs continue to be free of metastasis
and fibrosis.
Discussion
The approach to treatment of this case of
osteosarcoma in a dog was an experimental
one. Part of the reason for this approach was
the need to select a route of therapy designed
to preserve the limb, as the owner did not
wish the leg to be amputated. Another reason
was Drs. Grier's and Runyon's desire to
evaluate combination therapy with limb
preservation in mind, as described in recent
literature. 28
The particular combination of bleomycin,
local hyperthermia and levamisole was
derived with certain advantages in mind and
hopefully, a minimal number of disad-
vantages.
The principle reason bleomycin was
selected as the chemotherapeutic agent was its
synergistic effect with local hyperthermia. 18
Also, in mice this drug has been found to
concentrate in the lungs, along with skin,
kidneys, peritoneum and lymphatics. I Since
metastasis to the lungs is a major concern with
osteosarcoma, it was hoped to use this to an
advantage. The major disadvantages of
bleomycin were cost, at $157.00 per 15 units,
and the possible side effect of pulmonary
fibrosis. Thus far pulmonary fibrosis has not
been detected on radiographs of this dog.
Local hyperthermia was advantageous in
several ways. As noted previously, bleomycin
is markedly potentiated when administered
simultaneously with hyperthermia, suggesting
a true interaction. Results of simultaneous
combination therapy in mice were better than
either bleomycin or hyperthermia alone or
when given 24 hours apart. The main disad-
vantage is that bleomycin is enhanced
significantly only near 43° C, which is near
the top of the therapeutic range and leads to a
greater hazard of possible toxicity. 18
Another advantage of hyperthermia is
that it has been shown to increase the im-
munogenicity of some tumor cells, perhaps by
unburying some of the cell surface antigens
from surrounding lipids. 24
It also seems relevant that, as compared
to surgical removal (which eliminates poten-
tial antigens); and radiotherapy, chemo-
therapy, and whole body hyperthermia
(which suppresses antibody formation), local
hyperthermia may cause a slow release of an-
tigens with no inhibition, and possibly even
an increase, in antibody formation. 24
Problems possible with local hyperthermia
are cardiac arrhythmias, hepatic and renal
dysfunction, low grade fever due to necrosis,
and cutaneous burns. 24
Levamisole was used in this case in an at-
tempt to help restore the immune responses of
a predictably immunosuppressed dog.
Though the mechanism of action is unknown,
it is well understood that the best results are
obtained in immunodeficient patients. The
drug modulates immune function at 2 to 3
mg/kg of body weight. At higher doses, it
may actually suppress immune function. 8
Though the mechanism of action is un-
known, levamisole in vitro and levamisole
therapy in vivo correct defective motility in
phagocytic cells. The drug also stimulates
phagocytosis in cultured monocytes.8
Some immunodeficient patients do not
improve with levamisole treatment. It may be
due to the inability of the individual tp pro-
duce levamisole-induced serum factor needed
to increase lymphocyte funciton. 8 If the lym-
phocyte transformation test is accurate in its
assessment of the animal's immune status,
then the results of the levamisole therapy to
date is discouraging, as the dog continues to
be immunosuppressed.
The reliability of the lymphocyte transfor-
mation test is a controversial matter. Some
feel it is a good prognostic test, while others
do not.
9 The work on this project has assumed
the test to be reliable and will continue to do
so. There are very few good ways to assess im-
munostatus in such a quantified manner as
with this test.
To close this report no definite conclu-
sions can be drawn from this one clinical, ex-
perimental case that has yet to run its com-
plete course. Additional cases treated using
this therapeutic protocol, each individual
drug and other drugs as well as controls, are
needed to factually evaluate the results. This
will take a great deal of time, energy and
money and will require cooperation among
many researchers involved in cancer work.
References
1. Baker CE jr (publisher): Physicians' Desk Reference,
34th Edition. Litton Indust Inc, Oradell, Nj, pp.
706-707, 1980.
2. Banks WC, Morris E, Herron MR, Green RW:
Osteogenic~arcomaassociated with internal fixation
in two dogs. JAVMA 167:166-167, 1975.
3. Barrett jT: Textbook of Immunology. CV Mosby
Co, St Louis, 1970, p. 215.
4. Bennett D, Campbell jR, Brown P: Osteosarcoma
associated with healed fractures. J Sm A nim Pract
20:13-18,1979.
5. Brodey RS, AbtDA: Results of surgical treatment in
65 dogs with osteosarcoma. JA VMA 168:1032-1035,
1976.
6. Brodey RS, Riser WH: Canine osteosarcoma-A
clinicopathologic study of 194 cases. Clin Ortho and
Related Res 62:54-64,1969.
7. Brunner Cj, Muscoplat CC: Immunomodulatory
effects oflevamisole. JA VMA 176: 1159-1161, 1980.
9. Cochran AJ: In vitro testing of the immune response,
Immunological Aspects of Cancer. Castro jE (ed)
University Park Press, Baltimore, pp. 226-228,
1978.
10. Dorn CR: Epidemiology of canine and feline tumors.
JAAHA 12-307-312, 1976.
11. Feist jH: The biologic basis of radiologic findings in
bone disease. Radiol Clin N Amer8:183-205, 1970.
12. Friel jP (publisher): Dorland's Illustrated Medical
Dictionary, 25th Edition. WB
Saunders,
Philadelphia, p. 1379, 1974.
13. Gold jM, Freedman SO: Diagnostic tests in clinical
immunology, Clinical Immunology, Freedman SA
and Gold P (eds). Harper and Row, Hagerstown,
Md, p. 609,1976.
14. Hardy WD: The etiology of canine and feline
tumors. JAAHA 12:313-334, 1976.
15. Henness AM, Theilen GH, Park RD, Buhles WC:
Combination therapy for canine osteosarcoma. JA V-
MA 170:1076-1080, 1977.
16. Knecht CD, Priester W A: Musculoskeletal tumors in dogs. JA VMA 172:72-74, 1978.
17. Ling GV, Morgan jP, Pool RR: Primary bone
tumors in the dog: A
combined clinical,
radiographic and histologic approach to early
diagnosis. JA VMA 165:55-67,1974.
18. Marmor jB: Interactions of hyperthermia and
chemotherapy in animals. Cancer Research
39:2269-2276, june 1979.
19. Misdorp W, Hart AAM: Some prognostic and
epidemiologic factors in canine osteosarcomla. J Natl
Cancer Inst 62:537-545,1979.
20. Owen LN, Bostock DE, Lavelle RB: Studies on
therapy of osteosarcoma in dogs using BCG vaccine.
J Am Vet RadiolI8:27-29, 1977.
21. Peterson LFA, jones jM, Kelly Pj, Pease
GL:
Isolation of osteosarcoma cells from peripheral blood
after biopsy. Mayo Clin Proc 35:443-447,1960.
22. Pool RR: Tumors of bone and cartilage, Tumors in
Domestic Animals, 2nd ed. Moulton jE (ed),
Berkeley, University of California Press, pp. 89-149,
1978.
23. Schneider PR, Stowater jL: Pathologic fractures
associated with skeletal metastasis of osteosarcoma in
a dog. JAVMA 175:61-64, 1979.
24. Short jG: Hyperthermia and cancer: a brief review.
(a bulletin) BSD Corp, Salt Lake City, pp. 1-13,
1978.
25. Sinibaldi K, Rosen H, Liu SK, DeAngelis
M:
Tumors associated with metallic implants in
animals. Clin Ortho and Relat Res 118:257-266,
1976.
26. Smith HA, Jones, TC, Hunt RD:
Neoplasia,
Veterz"nary Pathology, 4th Edition. Lea and Febiger,
Philadelphia, pp 197-201,1972.
27. Theilen GH, Madewell BR: Tumors of the skeleton,
Veterz"nary Cancer Medz"cine. Lea and Febiger,
Philadelphia, pp 289-306,1979.
28. Theilen GH, Pool RR, Park RD: Treatment of
canine osteosarcoma for limb preservation using
osteotomy, adjuvant radiotherapy and
chemotherapy. VM/ SA C 72: 179-183, 1977.
29. Tjalma RA: Canine bone sarcoma: estimation of
relative risk as a function of body size. ] Natl Cancer
Inst36:1137-1150, 1966.
30. Wolke RE, Nielson SW: Site incidence of canine
osteosarcoma. ] 8m Anim Pract 7:489-492, 1966.
Share this article / Teilen Sie diesen Artikel
Diese Webseite wurde mit Jimdo erstellt! Jetzt kostenlos registrieren auf https://de.jimdo.com








