Invitrogen Life Technologies, Grand Island, NY

Article added / Artikel hinzugefügt 01.10.2021
Generally Articles and Discussions about Osteosarcoma in Dogs
→ Evaluations of phylogenetic proximity in a group of 67 dogs with
osteosarcoma: a pilot study
Article added / Artikel hinzugefügt 01.10.2021
Generally Articles and Discussions about Osteosarcoma in Dogs
→ Canine Periosteal Osteosarcoma
Images added / Abbildungen hinzugefügt 02.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Pulmonary vessels
New subcategory added / Neue Unterkategorie hinzugefügt 02.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Pulmonary vessels
Images added / Abbildungen hinzugefügt 01.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Heart valvular diseases



Association between Absolute Tumor Burden and Serum Bone-Specific Alkaline Phosphatase in Canine Appendicular Osteosarcoma

Sternberg, R.A., Pondenis, H.C., Yang, X., Mitchell, M.A., O'Brien, R.T., Garrett, L.D., Helferich, W.G., Hoffmann, W.E. and Fan, T.M. (2013), Association between Absolute Tumor Burden and Serum Bone-Specific
Alkaline Phosphatase in Canine Appendicular
Osteosarcoma. Journal of Veterinary Internal Medicine,
27: 955–963. doi: 10.1111/jvim.12121

Abstract
Background
In dogs with appendicular osteosarcoma (OSA), increased pretreatment serum bone-specific alkaline phosphatase (BALP) activity is a negative prognostic factor, associated with shorter disease-free intervals and survival times, but a biologic basis for observed differential serum BALP activities in canine OSA patients remains incompletely defined.
Objective
Serum BALP activity will correlate with absolute tumor burden in dogs with OSA.
Animals
This study included 96 client-owned dogs with appendicular OSA.
Methods
In canine OSA cell lines, the expression and membranous release of BALP was evaluated in vitro. The correlation between serum BALP activity and radiographic primary tumor size was evaluated in OSA-bearing dogs. In dogs developing visceral OSA metastases, serial changes in serum BALP activities were evaluated in relation to progression of macroscopic metastases, and visceral metastatic OSA cells were evaluated for BALP expression.
Results
In vitro, BALP expression was not associated with either tumorigenic or metastatic phenotype, rather the quantity of membranous BALP released was proportional with cell density. In dogs devoid of macroscopic metastases, there was a positive correlation between serum BALP activity and absolute primary tumor size. In dogs with progressive OSA metastases, serum BALP activity increased and coincided with the development of macroscopic metastases. OSA cells derived from visceral metastatic lesions retained BALP expression.
Conclusions and Clinical Importance
Tumor burden is a determinant of serum BALP activity in dogs with appendicular OSA. The association between increased pretreatment BALP activity and negative clinical prognosis may simply be attributed to greater initial tumor burden, and consequently more advanced tumor stage.
- ALP
-
alkaline phosphatase
- BALP
-
bone-specific alkaline phosphatase
- NTx
-
N-telopeptide
- OSA
-
osteosarcoma
- PI-PLC
-
phosphatidylinositol-specific phospholipase C
- rBMD
-
relative bone mineral density
- TALP
-
total alkaline phosphatase
Alkaline phosphatase (ALP) consists of a group of metalloenzymes that hydrolyze monophosphate esters at alkaline pH. There are 2 different isoenzymes of ALP, produced from different genes, and several different isoforms, derived from posttranslational modification in isoenzymes. The liver and bone isoforms, products of the tissue nonspecific ALP gene, comprise the majority of serum total ALP (TALP), whereas corticosteroid ALP, an intestinal ALP gene product, also contributes to TALP in dogs.[1] Bone ALP (BALP) is produced by osteoblasts and is anchored to the outer membrane surface by a hydrophobic glycosylphosphatidylinositol (GPI) linkage.[2] This type of membrane insertion permits the release of linked proteins, including BALP, from the outer membrane by the action of 1 or more GPI-specific phospholipases.[3]
In both dogs and humans with appendicular osteosarcoma (OSA), increased pretreatment serum TALP and BALP activities are negative prognostic factors, associated with shorter disease-free intervals and survival times.[4-11] Because of the restricted cellular source of BALP, direct assessment of BALP activity might serve as a more accurate biomarker than TALP in the evaluation and monitoring of patients with OSA. Several biologically based theories have been proposed to explain the variability in BALP activities among individuals with OSA, including (1) differences in rate of enzyme production or release by malignant osteoblasts, (2) differences in degree and extent of host reparative osteoblastic responses, and (3) variability in malignant osteoblast tumor burden.
In human OSA patients, a positive correlation between serum BALP activity and absolute tumor volume has been identified, with absolute tumor volume being a predictor of metastasis-free survival and overall survival.[12-14] In addition, patients with metastatic OSA have significantly higher serum BALP activity than those with nonmetastatic OSA, suggesting that overall tumor burden contributes to serum BALP.[12, 15] Despite the definitive relationships observed for serum BALP activity, OSA burden, and clinical outcome in people, the biologic basis for the correlation between BALP activities and prognosis in OSA-bearing dogs remains speculative and incompletely defined.
The purpose of this study was to investigate plausible biologic foundations underlying the prognostic relevance of serum BALP activities in canine appendicular OSA. The objectives of this study were as follows: (1) to evaluate the contributions of inherent biologic phenotype and absolute cell number on BALP expression in canine OSA cell lines in vitro, and (2) to evaluate the relationship between serum BALP activities and various tumor parameters in dogs with appendicular OSA. We hypothesized that serum BALP is a surrogate of malignant osteoblast numbers and would correlate with absolute tumor burden in OSA-bearing dogs.
Materials and Methods
In Vitro Studies
Cell Lines
Five canine OSA cell lines including the HMPOS (provided by Dr James Farese, University of Florida) and the KOS series 001-004 (provided by Dr Chand Khanna, National Cancer Institute) were evaluated for BALP expression. All cell lines were cultured in Dulbecco's Modified Eagle Medium (DMEM) with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Cell cultures were maintained at 37°C in 5% CO2 and passaged twice weekly.
Primary Osteoblast Isolation and Culture
Bone fragments were obtained from the proximal humeri of 4 skeletally normal dogs submitted to the University of Illinois Veterinary Diagnostic Laboratory for necropsy in good postmortem condition. Care was taken to obtain samples from the metaphyseal region predominantly composed of metabolically active trabecular bone. Briefly, bone fragments of about 2 mm3 were washed with PBS 3 times and digested in 2 mg/mL type IV collagenase. The enzymatic reaction was stopped by adding an equal volume of DMEM with 10% FBS. Bone fragments were cultured in 100 mm culture dishes in DMEM with 10% FBS. After migration of cells off bone fragments and onto the dish, cells were maintained in modified osteoblast differentiation medium[16] (DMEM with 10% FBS, 1% Pen/Strep, 0.2 mM ascorbic acid, 10 mM beta-glycerol phosphate, and 1 μM dexamethasone), which was changed 3 times weekly until cells were harvested.
PI-PLC Induced Release of BALP from Cell Membranes
HMPOS cells were harvested and pelleted in microcentrifuge tubes at counts of 4 × 106, 2 × 106, 1 × 106, and 5 × 105 per tube. Cells were washed with phosphate buffered saline (PBS) and resuspended in 0.5 mL PBS with or without 0.5 U phosphatidylinositol-specific phospholipase C (PI-PLC) isolated from Bacillus cereus1 for 30 minutes at 37°C. After incubation, cells were pelleted, the supernatant was collected for Western blot, and the cells were resuspended in media, plated on chamber well slides, and allowed to adhere for ALP staining.
Cytologic Detection of ALP Activity
Unstained slides of PI-PLC treated and untreated HMPOS cells were stained with nitroblue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate toluidine salt2 (NBT/BCIP), an ALP substrate, as previously reported.[17] Briefly, slides were incubated for 10 minutes at room temperature with sufficient amounts of NBT/BCIP to coat the slide. Slides were rinsed with water, blotted dry, and examined microscopically. Positive staining was indicated by brown to black staining of the cell surface.
Western Blot Analysis
Cellular proteins from OSA cell lines and canine osteoblasts were extracted with a commercial reagent3 and concentrations quantified with a standard assay kit.4 For the detection of total cellular BALP, 50 μg of protein was collected from each respective OSA and osteoblast cell line. For assessing the amount of membranous cleaved BALP released into cell culture media, a 20 μL aliquot of supernatant derived from varying HMPOS cell densities after PI-PLC incubation was used. Protein was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using 10% polyacrylamide gels and transferred onto a nitrocellulose membrane. After 1 hour blocking at room temperature in 5% nonfat dry milk PBS-Tween, the membrane was incubated with a rabbit monoclonal anti-human/mouse BALP antibody5 (1 : 1,000) in 5% nonfat dry milk PBS-Tween for 1 hour at room temperature. The membrane was washed 3 times and then incubated for 1 hour at room temperature with a horseradish peroxidase conjugated anti-rabbit secondary antibody (1 : 1,000) in 5% nonfat dry milk PBS-Tween and developed using a standard chemiluminescence detection kit.6 For subjective comparison of BALP expression among canine OSA and normal osteoblast cell lines, rabbit anti-β-actin polyclonal antibody5 (1 : 5,000) was used to ensure equivalent loading of protein in separate wells.
Canine Studies
Study Population
The study included dogs with OSA that were evaluated at the University of Illinois Cancer Care Clinic between 2003 and 2012. All dogs had a diagnosis of appendicular OSA confirmed by either histopathology or cytology with concurrent positive ALP staining.[17] All dogs included in the study had orthogonal radiographs of the primary tumor, 3-view thoracic radiographs, DEXA scan, and urine and serum collected at the time of diagnosis. Study candidates were excluded if they had previously received other forms of treatment including radiation treatment, cytotoxic chemotherapy, or IV bisphosphonates and if they had radiographically apparent pulmonary metastases or metastases to other distant sites at the time of diagnosis. After their initial visits, all dogs were treated palliatively for OSA, receiving coarse-fraction radiation treatment with or without an IV aminobisphosphonate. Dogs were re-evaluated at 28-day intervals, at which time serum and urine were collected. When owner consent was given, necropsy was performed at the time of euthanasia.
Serum BALP
Serum was collected at the time of initial presentation, and samples were stored at −80°C until analysis. Serum BALP was evaluated with a commercial ELISA7 test kit utilizing a murine monoclonal anti-BALP antibody, previously validated for use in dogs.[18] Reference range for dogs ≥2 years of age is 0–14 U/L (reflects mean activity ±2 standard deviations).[18]
Urine NTx Excretion
Urine was collected (voided or cystocentesis) at the time of initial presentation. Urine samples were immediately centrifuged at 4°C, 400 × g for 10 minutes, and the supernatant was collected and stored at −80°C until analysis. Urine NTx concentrations were measured with a commercial ELISA8 test kit, previously validated for use in dogs[19, 20] and expressed as normalized nanomolar (nM) bone collagen equivalents (BCE) per millimolar (mM) urinary concentration of creatinine.
Radiography
Anterior-posterior (AP) and lateromedial radiographic views of affected limbs were evaluated and interpreted separately for each dog by 1 investigator (RAS). Radiographic assessment of tumor involvement was determined based on the following characteristic changes in bone: lysis of cortical or medullary bone, sclerosis, and periosteal new bone formation.[21]
Calculation of Tumor Size
The following size parameters similar to those defined by Bieling et al[14] were determined from each dog's radiographic images:
- Absolute tumor length (ATL): the greater longitudinal extension of the tumor measured on either AP or lateral view (cm).
- Absolute tumor width (ATW): horizontal tumor extension measured on the AP view.
- Absolute tumor depth (ATD): horizontal tumor extension measured on the lateral view.
- Absolute tumor plane (ATP): the maximal cross-sectional area calculated from the tumor length and the larger of the 2 diameters (width or depth),
designated Max [ATW, ATD], by the ellipse formula:
-
- Relative tumor plane (RTP): ATP divided by body surface area (BSA, cm2/m2).
- Absolute tumor volume (ATV): calculated from the 3 parameters length, width, and depth, by the ellipsoid formula:
-
- Relative tumor volume (RTV): ATV divided by BSA (cm3/m2).
- Absolute tumor area (ATA): the cross-sectional area calculated from the tumor width and depth by the ellipse formula:
-
- Relative tumor area (RTA): ATA divided by BSA (cm2/m2).
- Absolute tumor surface area (ATSA): the surface area calculated from the tumor width, depth, and length by the approximate ellipsoid surface area
formula[22, 23] (Knud Thomsen's formula):
where p = 1.6075 and a, b, and c represent the semiaxes with a < b < c.
-
- Relative tumor surface area (RTSA): ATSA divided by BSA (cm2/m2).
Relative Bone Mineral Density (rBMD) of Primary Tumor
At initial presentation, DEXA9 scans were performed to measure rBMD of the primary tumor and an equivalent anatomic area of the unaffected contralateral limb. Dogs were sedated and positioned in sternal or lateral recumbency for forelimb or hind limb lesions, respectively. Bone mineral density of the primary tumor was divided by BMD of the contralateral normal limb to determine rBMD as previously described.[24]
BALP Immunohistochemistry Antibody Validation
Normal canine kidney was used as a positive control for antibody validation and staining protocol optimization. Tissue was fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned at 5 μm. The protocol consisted of deparaffinization and rehydration of the sections through graded alcohol baths, endogenous peroxidase quenching with a methanol–hydrogen peroxidase solution, microwave antigen retrieval with citrate buffer (pH 6.0), and incubation in the primary antibody. Anti-BALP monoclonal antibody5 was diluted 1 : 500, incubated for 30 minutes, and detected with a non avidin-biotin immunoperoxidase- diaminobenzidine (DAB) detection method.10 Primary antibody was omitted as a negative control.
BALP Immunohistochemistry of OSA Samples
Complete necropsy was performed on 8 dogs with not only primary bone OSA but also advanced macroscopic metastases involving distant visceral organs. Tissue specimens from the primary tumor and all major organs were submitted for histologic examination. All tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin (HE). Based on the HE-stained slides, additional paraffin sections were obtained from the primary tumors and selected soft tissue metastatic sites for BALP immunohistochemistry.
Statistical Analysis
The distribution of the continuous variable data was evaluated using the Shapiro–Wilk test, skewness, kurtosis, and q–q plots. Data that were not normally distributed were log transformed to meet the assumption of normality. A Pearson product-moment correlation coefficient was used to assess the relationship between pretreatment serum BALP and tumor parameters (measurements of tumor size, urine NTx, and rBMD). Changes in BALP and urine NTx over time were evaluated with repeated measures ANOVA followed by posthoc paired 2-tailed t-tests for normally distributed data (BALP) or by Wilcoxon matched-pairs signed-ranks test for nonparametric data (urine NTx). Statistical calculations were performed using commercial software programs.11,12 P < .05 was considered statistically significant for all analyses.
Results
In Vitro Studies
Characterization of BALP Expression Relative to OSA Biologic Behavior and Cell Number
We first examined if expression of BALP correlated with the biologic properties of cells of osteoblast lineage, specifically in nonmalignant primary culture osteoblasts and immortalized malignant osteoblasts possessing variable in vivo tumorigenic and metastatic potential. BALP expression was detected by Western blot analysis (~75 kDa) in all nonmalignant and malignant canine osteoblast cell lines evaluated (Fig 1A). Expression of BALP was robust and relatively consistent in all 4 nonmalignant osteoblast cell lines. With respect to the 4 malignant OSA cell lines, BALP was expressed by all 4 lines, but there was decreased expression of BALP in 2 cell lines (lanes 7 and 8) that previously have been characterized to have the greatest tumorigenicity and metastatic potential in vivo.[25]
Figure 1. (A) Bone-specific alkaline phosphatase (BALP) is expressed in all normal canine osteoblast (lanes 1–4) and KOS osteosarcoma (OSA) (lanes 5–8) cell lines as demonstrated by Western blot analysis. BALP expression is weakest in the most biologically aggressive OSA cell lines when normalized for β-actin loading control. (B) BALP was cleaved from the surface of HMPOS OSA cells and detected in the supernatant by western blot analysis. The amount of BALP cleaved decreases with decreasing cell number. (C) After phosphatidylinositol-specific phospholipase C (PI-PLC)-induced cleavage of BALP from the cell membrane, the cells demonstrated decreased alkaline phosphatase (ALP) activity (right panel), as shown by a decrease in NBT/BCIP (diffuse brown cytoplasmic) staining. Untreated HMPOS cells retaining high ALP activity serve as control (left panel).
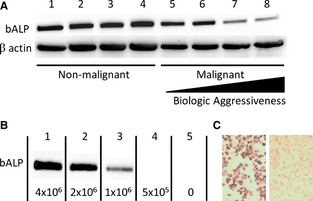
We next examined if the amount of membranous BALP available for release into serum correlated with OSA cell density. BALP was easily cleaved from the surface of HMPOS OSA cells by PI-PLC, and the amount of BALP released from the outer membrane decreased with decreasing cell number (Fig 1B). Confirming that PI-PLC incubation promoted cleavage of BALP from the cell membrane, adherent HMPOS cells incubated with PI-PLC had decreased ALP activity compared to untreated control cells, as shown via NBT/BCIP staining (Fig 1C).
Canine Studies
To determine if a relationship existed between serum BALP and absolute OSA burden in dogs, we evaluated whether or not there was a direct correlation between serum BALP and various primary tumor size parameters in dogs with appendicular OSA devoid of macroscopic metastases at the time of diagnosis.
Patient Characteristics
Ninety-six dogs with appendicular OSA were included in this study. There were 15 mixed breed dogs and 81 purebred dogs. The breeds represented included Rottweiler (n = 15), Great Dane (n = 9), Labrador Retriever (n = 9), Saint Bernard (n = 8), Golden Retriever (n = 7), Greyhound (n = 4), Irish Wolfhound (n = 4), Mastiff (n = 4), Doberman Pinscher (n = 3), German Shepherd (n = 3), Newfoundland (n = 3), Alaskan Malamute (n = 2), American Pit Bull (n = 2), Bernese Mountain Dog (n = 2), Great Pyrenees (n = 2), and 1 of each of the following breeds: Australian Shepherd, Chesapeake Bay Retriever, Dalmatian, and Old English Sheepdog. There were 38 female (37 spayed) and 58 male (55 castrated) dogs. The mean and median ages were both 7.8 years (range, 2.5–12.6 years). The mean and median weights were 48.2 and 44.1 kg, respectively (range, 25.8–84.1 kg).
Tumor Characteristics
Tumors were located in the distal radius (n = 48), proximal humerus (n = 16), distal femur (n = 10), distal tibia (n = 9) proximal tibia (n = 5), proximal femur (n = 3), distal ulna (n = 2), distal humerus (n = 2), and proximal radius (n = 1).
Relationship between Serum BALP and Tumor Parameters
The median serum BALP concentration was 21.3 U/L (range, 4.7–123.3 U/L) and the median tumor parameters including tumor length, ATSA, and ATV were 7.4 cm (range, 2.7–16.0 cm), 69.5 cm2 (range, 13.0–217.9 cm2), and 45.4 cm3 (range, 4.1–237.4 cm3), respectively. There was a positive correlation between log BALP and the following tumor variables: (1) tumor length (r = 0.646, n = 96, P < .0001), (2) ATSA (r = 0.662, n = 96, P < .0001), and (3) ATV (r = 0.621, n = 96, P < .001). Scatter plots summarizing the results are shown in Figure 2. Correlations were weaker, but maintained significance, between log BALP and relative measures of tumor size. The mean and median for rBMD and urine NTx were 1.0 ± 0.5 and 225.8 nM BCE/mM creatinine (range, 52.6–645.2 nM BCE/mM creatinine), respectively. No association was identified between log BALP and rBMD or log BALP and urine NTx (data not shown).

Figure 2. Scatterplots depicting the significant correlation between log serum bone-specific alkaline phosphatase and (A) tumor length, (B) absolute tumor surface area, and (C) absolute tumor volume, calculated from radiographic measurements of the primary tumor, in 96 canine appendicular osteosarcoma patients.
Changes in BALP and NTx with OSA Treatment and Progression
Serial changes in serum BALP and urine NTx were evaluated in 8 dogs undergoing palliative treatment for OSA with subsequent development of histologically confirmed distant metastatic disease. Serum and urine were analyzed at the time of diagnosis, 28 days after starting palliative treatment and at the time of euthanasia attributable to advanced macroscopic metastatic disease burden. For the subset of dogs evaluated, differences in serum BALP activity were significant (P = .009). In posthoc analysis, there was a significant decrease in serum BALP activity between baseline and day 28 (64.9 ± 19.7 U/L and 25.3 ± 8.0 U/L, respectively; P = .05), a significant increase in serum BALP activity between day 28 and terminal (25.3 ± 8.0 U/L and 232.7 ± 81.3 U/L, respectively; P = .03), and a significant increase in serum BALP activity between baseline and terminal (P = .04; Fig 3A). For the subset of dogs evaluated, differences in urine NTx concentrations were significant (P = .002). There was a significant decrease in urine NTx between baseline and day 28 (167.0 nM BCE/mM creatinine [range, 113.8–352.2 nM BCE/mM creatinine] and 33.1 nM BCE/mM creatinine [range, 19.2–226.8 nM BCE/mM creatinine]), respectively, (P = .008) and between baseline and terminal (167.0 nM BCE/mM creatinine [range, 113.8–352.2 nM BCE/mM creatinine] and 45.8 nM BCE/mM creatinine [range, 28.6–246.9 nM BCE/mM creatinine]), (P = .02) measurements, respectively. There was no significant difference between day 28 and terminal urine NTx measurements (P = .08; Fig 3B).

Figure 3. Serial quantification of (A) serum bone-specific alkaline phosphatase (BALP) activity and (B) urine N-telopeptide (NTx) from 8 dogs with appendicular osteosarcoma assessed at the time of diagnosis, 28 days after initiation of palliative treatment, and at the time of euthanasia with distant metastatic disease. BALP activity is expressed as mean ± SD, whereas urine NTx is expressed as median (±min–max range). *Indicates a significant difference defined as P < .05.
BALP Immunohistochemistry
From the 8 dogs with histologically confirmed measurable metastatic disease, we selected 3 dogs that had metastasis to various soft tissue sites and performed BALP IHC on those tissues. Malignant OSA cells at all of the distant metastatic sites, including kidney, liver, spleen, sternal lymph node, lung, and mediastinum, exhibited strong BALP immunopositivity, similar to parental OSA cells derived from the original primary tumor sites (Fig 4).

Figure 4. Osteosarcoma (OSA) lesions from 3 dogs with metastatic appendicular OSA. Hematoxylin and eosin (HE) staining of the (A) humerus and bone-specific alkaline phosphatase (BALP) immunohistochemistry (IHC) of the (B) humerus, (C) kidney, and (D) liver from dog 1. HE staining of the (E) radius and BALP IHC of the (F) radius, (G) spleen, and (H) sternal lymph node from dog 2. HE staining of the (I) radius and BALP IHC of the (J) radius, (K) lung, and (L) mediastinum from dog 3. Immunopositive cells exhibit strong, diffuse, cytoplasmic staining via DAB chromogen detection.
Discussion
Results of this study suggest that differences in absolute tumor burden, and thus OSA cell number, contribute to the observed differential serum BALP activities in dogs with OSA. The contribution of absolute OSA cell density to serum BALP activity is supported by in vitro findings demonstrating that the amount of cleavable membranous BALP, which is potentially available for serologic detection, increased with higher OSA cell numbers. Corroborating the cell line data, in dogs with nonmetastatic appendicular OSA, there was a strong, positive correlation between serum BALP activity and 1-, 2-, and 3-dimensional absolute measurements of primary tumor size. In addition, OSA cells derived from visceral metastases retained BALP expression, and dogs with metastatic disease had a significant increase in serum BALP activity coinciding temporally with development of macroscopic soft tissue metastases. Because larger tumors, either primary bone or secondary metastases, hypothetically are composed of a higher number of neoplastic cells, the positive correlation identified in this study between tumor burden and BALP activity lends support to the hypothesis that OSA cell number is a biologic determinant of serum BALP activity.
An interesting finding in this study was that in vitro BALP expression did not correlate with the in vivo biologic aggressiveness of the canine osteoblast cell lines. In normal osteoblasts, the expression of BALP was robust in all 4 primary osteoblast cultures, and BALP expression was subjectively more intense compared to the 4 malignant OSA cell lines. With regard to the 4 characterized canine OSA cell lines (K001-K004), inherent differences in BALP expression was identifiable. Specifically, BALP was most weakly expressed by K003 cells (Fig 1A, lane 8); however, with respect to the K001-004 cell line series, K003 is the most tumorigenic cell line in vivo, and the only cell line that is spontaneously metastatic.
Based on the normal and malignant osteoblast cell lines used in this investigation, the data suggest that higher BALP expression might not be linked to greater tumorigenic or metastatic cellular behavior. To date, few studies have investigated putative cellular mechanisms, such as differential signaling pathways or enzymatic activities that might regulate serum TALP or BALP expression in OSA patients. Recently, the Wnt/β-catenin signaling pathway has been demonstrated to participate in bone biology by induction of ALP in pre-osteoblastic cells. However, in dogs with OSA, differential activity in the Wnt/β-catenin signaling pathway did not account for differences in serum TALP activity.[26] In another pilot investigation13 utilizing isogenic paired OSA cell lines with divergent metastatic potential, enzymatic activity of phospholipase D2 was correlated with capacity for membranous BALP cleavage. Although highly metastatic variants demonstrated greater phospholipase D2 enzymatic activity, the rate of BALP cleavage did not correlate with phospholipase D2, and the highest BALP activity was not consistently identified in the most metastatic isogenic cell line pairs. Based on these preliminary negative results, additional investigations are necessary to further characterize inherent molecular determinants that might contribute to BALP expression in OSA cells.
Increased serum BALP activity is not a unique characteristic of OSA and is commonly seen with bone reparative and physiologic processes such as young age,[18] fracture healing,[27, 28] osteoporosis,[29] Paget's disease,[30] hyperparathyroidism,[31] thyrotoxicosis,[32] and bone metastasis from prostate or breast cancer.[33, 34] This observation raises the possibility that increased serum BALP activity in dogs with OSA is secondary not only to malignant osteoblasts but also to a general reparative osteoblast response associated with focal malignant osteolysis. To quantify the rate and extent of bone turnover, and thus the potential contribution of general skeletal remodeling to serum BALP activity in OSA-bearing dogs, we quantified urine N-telopeptide (NTx) concentrations at 3 serial time points (day 0, day 28, and terminal) in dogs with nonmetastatic OSA that underwent palliative treatment and subsequently developed visceral metastatic disease. Urine NTx decreased significantly within 28 days of starting palliative treatment in these OSA-bearing dogs, suggesting that malignant osteolysis is effectively arrested within the primary bone tumor. Coinciding with decreases in urine NTx, serum BALP activity also decreased significantly, a consequence of decreased viability and activity of malignant OSA cells and resident reparative osteoblasts. However, in dogs developing terminal macroscopic soft tissue metastases, urine NTx remained low whereas serum BALP activity increased significantly. The divergence of urine NTx and BALP activity in dogs with terminal macroscopic extraskeletal metastases suggests that proliferating OSA cells, and not activated reparative osteoblasts at the level of the primary tumor or occult bone metastatic sites, are the major source of serum BALP activity.
To further substantiate that metastatic OSA cells proliferating within soft tissue visceral organs likely contribute to serum BALP activity, primary bone tumors and soft tissue visceral metastases were harvested from terminal OSA-bearing dogs after humane euthanasia and stained for BALP expression. All OSA cells derived from both the primary bone tumor and visceral metastases of various organs stained strongly for BALP. The uniform BALP staining of OSA cells, regardless of origin, lends additional credence to the hypothesis that macroscopic OSA burden has the capacity to directly contribute to serum BALP activity.
Additional evidence for absolute OSA tumor burden as the primary predictor of serum ALP activity can be found in other OSA models in the literature. A study by Ghanta et al[35] evaluated an orthotopic, syngeneic murine model of OSA, which recapitulates the natural progression of disease in that mice that underwent amputation surgery remained disease-free at the primary tumor site but subsequently developed spontaneous pulmonary metastatic disease. In this study, mice developed significant increases in serum TALP activity at the time of development of pulmonary metastases, and the extent of increased serum TALP activity correlated with the size and weight of the pulmonary metastases. In human OSA patients, serum BALP activity appears to be a sensitive biochemical marker for monitoring OSA recurrence and progression, because increases in serum BALP activity precede clinical or radiographic evidence of disease recurrence by up to 3 weeks.[15]
Initial tumor size is a well-established prognostic factor in human OSA patients.[13, 14, 36] Attributable to the positive correlation found between initial tumor size and serum BALP activity in this study, the results suggest that tumor size may also be a prognostic factor in dogs with OSA, and these study findings raise potentially important clinical implications. Because OSA cells continue to express BALP at metastatic sites (thus retaining ability to contribute to serum BALP activity), more sensitive staging tests should be considered in patients with increased serum BALP activity at the time of diagnosis or with progressive increases in BALP activity in the face of treatment. Thoracic radiography, which is traditionally used in the staging of OSA patients, is an insensitive test, failing to detect up to 90% of pulmonary metastatic nodules when compared to CT imaging in dogs with metastatic cancer. The lower size limit of nodule detection on radiographs is 7–9 mm compared to 1 mm on CT images.[37] In addition, thoracic radiographs appear to be particularly poor at detecting pulmonary metastases in dogs with OSA, which may be related to the often irregular, poorly marginated borders of the metastatic lesions.[37, 38] Thoracic CT is used routinely in the staging of human OSA patients, and CT image findings have prognostic value. Although the size of lung metastases detected with CT is not prognostic for survival in pediatric OSA patients, number, distribution, timing (synchronous versus metachronous), and location (peripheral versus central) of metastases is predictive of outcome.[39, 40]
There are limitations to this study that should be addressed. First, the tumor size parameters were calculated from the dimensions measured on plain radiographs using simplified mathematical formulas rather than by direct 3-dimensional volume measurements. This is potentially problematic because tumors tend to have an irregular shape and heterogeneous composition. In human OSA patients, 3-dimensional magnetic resonance imaging (MRI) measurement provides the most reproducible measurements of tumor volume.[41, 42] In the veterinary literature, imaging studies primarily focus on identifying the modality that most accurately predicts tumor length for planning of limb-salvage procedures. These studies report conflicting results with regard to the utility of MRI in estimating tumor length. Radiographs, however, appear to reasonably approximate tumor extent and are more accurate than nuclear scintigraphy when using histopathology as the gold standard.[21, 43-45] Second, there was variability in the time of sample storage, with some serum and urine samples archived for years before analysis. Although this may have impacted some results, a previous study showed good correlation between ALP activities measured using fresh and frozen serum and stability of NTx in urine over time after freezing.[9, 46] Also, reference range for BALP activity used in this study (0–14 U/L) was different from that used in previous studies (approximately 3–23 U/L).[8, 9] This is attributable to differences in the techniques used to quantify BALP activity. In this study, an immunoassay was used to directly measure BALP activity.[18] In previous studies, an enzymatic assay (wheat germ-lectin precipitation/levamisole inhibition assay), which has some cross-reactivity with liver ALP, was used to measure BALP activity.[8, 9, 47] However, excellent correlation has been demonstrated between results obtained from the enzymatic and immunologic assays.[18] Lastly, the focus of this study was narrow, and only addressed the potential role of tumor burden on BALP activity in dogs with OSA. Additional host- or tumor-related factors, independent of tumor size, also may contribute to serum BALP activity, and future studies will be required to characterize other potential operative molecular mechanisms.
Despite these limitations, this study provides new information and supports a simple biologic basis for the observed differential serum BALP activities in dogs with appendicular OSA, specifically absolute tumor burden. Importantly, findings from this study highlight the need for clinical vigilance in assessing dogs that present with increased pretreatment BALP activity. For this subset of dogs, conventional radiography might underestimate disease burden, and more accurate tumor staging, and hence outcome stratification, might be better achieved with advanced imaging modalities such as CT.
Acknowledgments
The authors thank the veterinary oncology technicians, veterinary oncology residents, and oncology faculty of the University of Illinois Cancer Care Clinic for their contributions to this study.
Conflict of Interest Declaration: Dr Timothy M. Fan is an Associate Editor at the Journal of Veterinary Internal Medicine.
-
1
-
2
Kirkegaard and Perry Laboratories, Inc, Gaithersburg, MD
-
3
M-PER; Pierce, Rockford, IL
-
4
Bicinchoninic Acid Protein Assay; Pierce
-
5
Abcam, Cambridge, MA
-
6
ECL Kit; GE Healthcare Life Sciences, Piscataway, NJ
-
7
MicroVue BAP EIA kit; Quidel, San Diego, CA
-
8
Osteomark; Wampole Laboritories, Princeton, NJ
-
9
QDR-4500W; Hologic, Bedford, MA
-
10
Biogenex Laboratories, Fremont, CA
-
11
GraphPad Prism, version 5 for Windows; GraphPad Software, San Diego, CA
-
12
MedCalc 12.0; MedCalc Software, Mariakerke, Belgium
-
13
Neumann Z, Pondenis H, Wypij J et al. Investigating phospholipase D2 expression in canine osteosarcoma. Veterinary Cancer Society 29th Annual Conference, Austin, TX, October 19, 2009
References
-
1
Syakalima M, Takiguchi M, Yasuda J, Hashimoto A. The canine alkaline phosphatases: A review of the isoenzymes in serum, analytical methods and their diagnostic application. Jpn J Vet Res 1998;46:3–11.
-
2
Magnusson P, Sharp CA, Farley JR. Different distributions of human bone alkaline phosphatase isoforms in serum and bone tissue extracts. Clin Chim Acta 2002;325:59–70.
-
3
Harrison G, Shapiro IM, Golub EE. The phosphatidylinositol-glycolipid anchor on alkaline phosphatase facilitates mineralization initiation in vitro. J Bone Miner Res 1995;10:568–573.Direct Link:
-
4
Bacci G, Picci P, Orlandi M, et al. Prognostic value of serum alkaline phosphatase in osteosarcoma. Tumori 1987;73:331–336.
-
5
Bacci G, Picci P, Ferrari S, et al. Prognostic significance of serum alkaline phosphatase measurements in patients with osteosarcoma treated with adjuvant or neoadjuvant chemotherapy. Cancer 1993;71:1224–1230.Direct Link:
-
6
Bacci G, Longhi A, Ferrari S, et al. Prognostic significance of serum alkaline phosphatase in osteosarcoma of the extremity treated with neoadjuvant chemotherapy: Recent experience at Rizzoli Institute. Oncol Rep 2002;9:171–175.
-
7
Bacci G, Longhi A, Versari M, et al. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer 2006;106:1154–1161.
-
8
Ehrhart N, Dernell WS, Hoffmann WE, et al. Prognostic importance of alkaline phosphatase activity in serum from dogs with appendicular osteosarcoma: 75 cases (1990–1996). J Am Vet Med Assoc 1998;213:1002–1006.
-
9
Garzotto CK, Berg J, Hoffmann WE, Rand WM. Prognostic significance of serum alkaline phosphatase activity in canine appendicular osteosarcoma. J Vet Intern Med 2000;14:587–592.Direct Link:
-
10
Moore AS, Dernell WS, Ogilvie GK, et al. Doxorubicin and BAY 12–9566 for the treatment of osteosarcoma in dogs: A randomized, double-blind, placebo-controlled study. J Vet Intern Med 2007;21:783–790.Direct Link:
-
11
Kirpensteijn J, Kik M, Rutteman GR, Teske E. Prognostic significance of a new histologic grading system for canine osteosarcoma. Vet Pathol 2002;39:240–246.
-
12
Limmahakhun S, Pothacharoen P, Theera-Umpon N, et al. Relationships between serum biomarker levels and clinical presentation of human osteosarcomas. Asian Pac J Cancer Prev 2011;12:1717–1722.
-
13
Kaste SC, Liu T, Billups CA, et al. Tumor size as a predictor of outcome in pediatric non-metastatic osteosarcoma of the extremity. Pediatr Blood Cancer 2004;43:723–728.
-
14
Bieling P, Rehan N, Winkler P, et al. Tumor size and prognosis in aggressively treated osteosarcoma. J Clin Oncol 1996;14:848–858.
-
15
Liu PP, Leung KS, Kumta SM, et al. Bone-specific alkaline phosphatase in plasma as tumour marker for osteosarcoma. Oncology 1996;53:275–280.
-
16
Barberi T, Willis LM, Socci ND, Studer L. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med 2005;2:e161.
-
17
Barger A, Graca R, Bailey K, et al. Use of alkaline phosphatase staining to differentiate canine osteosarcoma from other vimentin-positive tumors. Vet Pathol 2005;42:161–165.
-
18
Allen LC, Allen MJ, Breur GJ, et al. A comparison of two techniques for the determination of serum bone-specific alkaline phosphatase activity in dogs. Res Vet Sci 2000;68:231–235.
-
19
Allen MJ, Allen LC, Hoffmann WE, et al. Urinary markers of type I collagen degradation in the dog. Res Vet Sci 2000;69:123–127.
-
20
Ladlow JF, Hoffmann WE, Breur GJ, et al. Biological variability in serum and urinary indices of bone formation and resorption in dogs. Calcif Tissue Int 2002;70:186–193.
-
21
Davis GJ, Kapatkin AS, Craig LE, et al. Comparison of radiography, computed tomography, and magnetic resonance imaging for evaluation of appendicular osteosarcoma in dogs. J Am Vet Med Assoc 2002;220:1171–1176.
-
22
Klamkin MS. Elementary approximations to the area of N-dimensional ellipsoids. Am Math Monthly 1971;78:280–283.
-
23
Klamkin MS. Corrections to “Elementary approximations to the area of N-dimensional ellipsoids”. Am Math Monthly 1976;83:478.
-
24
Fan TM, de Lorimier LP, Charney SC, Hintermeister JG. Evaluation of intravenous pamidronate administration in 33 cancer-bearing dogs with primary or secondary bone involvement. J Vet Intern Med 2005;19:74–80.Direct Link:
-
25
Hong S-H, Osborne T, Ren L, et al. Protein kinase C regulates ezrin-radixin-moesin phosphorylation in canine osteosarcoma cells. Vet Comp Oncol 2011;9:207–218.
-
26
Piskun CM, Muthuswamy A, Huelsmeyer MK, et al. Wnt/β-catenin expression does not correlate with serum alkaline phosphatase concentration in canine osteosarcoma patients. PLoS One 2011;6(10):e26106.
-
27
Ikegami S, Kamimura M, Nakagawa H, et al. Comparison in bone turnover markers during early healing of femoral neck fracture and trochanteric fracture in elderly patients. Orthop Rev (Pavia) 2009;1:e21.
-
28
Komnenou A, Karayannopoulou M, Polizopoulou ZS, et al. Correlation of serum alkaline phosphatase activity with the healing process of long bone fractures in dogs. Vet Clin Pathol 2005;34:35–38.
-
29
Biver E, Chopin F, Coiffier G, et al. Bone turnover markers for osteoporotic status assessment? A systematic review of their diagnosis value at baseline in osteoporosis. Joint Bone Spine 2012;79:20–25.
-
30
Magnusson P, Davie MWJ, Sharp CA. Circulating and tissue-derived isoforms of bone alkaline phosphatase in Paget's disease of bone. Scand J Clin Lab Invest 2010;70:128–135.
-
31
Kerschan-Schindl K, Riss P, Krestan C, et al. Bone metabolism in patients with primary hyperparathyroidism before and after surgery. Horm Metab Res 2012;44:476–481.
-
32
El Hadidy EHM, Ghonaim M, El Gawad SSA, El Atta MA. Impact of severity, duration, and etiology of hyperthyroidism on bone turnover markers and bone mineral density in men. BMC Endocr Disord 2011;11:15.
-
33
Kamiya N, Suzuki H, Yano M, et al. Implications of serum bone turnover markers in prostate cancer patients with bone metastasis. Urology 2010;75:1446–1451.
- 34
-
35
Ghanta VK, Hiramoto RN, Weiss AB, Caudill L. Monitoring of murine osteosarcoma by serial alkaline phosphatase determinations. J Natl Cancer Inst 1976;57:837–839.
-
36
Kim MS, Lee S-Y, Cho WH, et al. Initial tumor size predicts histologic response and survival in localized osteosarcoma patients. J Surg Oncol 2008;97:456–461.Direct Link:
-
37
Nemanic S, London CA, Wisner ER. Comparison of thoracic radiographs and single breath-hold helical CT for detection of pulmonary nodules in dogs with metastatic neoplasia. J Vet Intern Med 2006;20:508–515.Direct Link:
-
38
Armbrust LJ, Biller DS, Bamford A, et al. Comparison of three-view thoracic radiography and computed tomography for detection of pulmonary nodules in dogs with neoplasia. J Am Vet Med Assoc 2012;240:1088–1094.
-
39
Rasalkar DD, Chu WCW, Lee V, et al. Pulmonary metastases in children with osteosarcoma: Characteristics and impact on patient survival. Pediatr Radiol 2011;41:227–236.
-
40
Letourneau PA, Xiao L, Harting MT, et al. Location of pulmonary metastasis in pediatric osteosarcoma is predictive of outcome. J Pediatr Surg 2011;46:1333–1337.
-
41
Shin K-H, Moon S-H, Suh J-S, Yang W-I. Tumor volume change as a predictor of chemotherapeutic response in osteosarcoma. Clin Orthop Relat Res 2000 Jul;(376):200–208.
-
42
Moon S-H, Shin K-H, Suh J-S, et al. Tumor volume change after chemotheraphy as a predictive factor of disease free survival for osteosarcoma. Yonsei Med J 2005;46:119–124.
-
43
Wallack ST, Wisner ER, Werner JA, et al. Accuracy of magnetic resonance imaging for estimating intramedullary osteosarcoma extent in pre-operative planning of canine limb-salvage procedures. Vet Radiol Ultrasound 2002;43:432–441.Direct Link:
-
44
Leibman NF, Kuntz CA, Steyn PF, et al. Accuracy of radiography, nuclear scintigraphy, and histopathology for determining the proximal extent of distal radius osteosarcoma in dogs. Vet Surg 2001;30:240–245.
-
45
Lamb CR, Berg J, Bengtson AE. Preoperative measurement of canine primary bone tumors, using radiography and bone scintigraphy. J Am Vet Med Assoc 1990;196:1474–1478.
-
46
Lomeo A, Bolner A. Stability of several biochemical markers of bone metabolism. Clin Chem 2000;46:1200–1202.
-
47
Sanecki RK, Hoffmann WE, Hansen R, Schaeffer DJ. Quantification of bone alkaline phosphatase in canine serum. Vet Clin Pathol 1993;22:17–23.Direct Link:
Share this article / Teilen Sie diesen Artikel
Diese Webseite wurde mit Jimdo erstellt! Jetzt kostenlos registrieren auf https://de.jimdo.com












