
Article added / Artikel hinzugefügt 01.10.2021
Generally Articles and Discussions about Osteosarcoma in Dogs
→ Evaluations of phylogenetic proximity in a group of 67 dogs with
osteosarcoma: a pilot study
Article added / Artikel hinzugefügt 01.10.2021
Generally Articles and Discussions about Osteosarcoma in Dogs
→ Canine Periosteal Osteosarcoma
Images added / Abbildungen hinzugefügt 02.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Pulmonary vessels
New subcategory added / Neue Unterkategorie hinzugefügt 02.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Pulmonary vessels
Images added / Abbildungen hinzugefügt 01.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Heart valvular diseases



Adjuvant Carboplatin and Gemcitabine Combination Chemotherapy Postamputation in Canine Appendicular Osteosarcoma

M. McMahon1, T. Mathie, N. Stingle, E. Romansik, D. Vail and C. London
Article first published online: 12 APR 2011
DOI: 10.1111/j.1939-1676.2011.0697.x

Abstract
Background:
Appendicular osteosarcoma (OSA), the most common bone tumor in dogs, is typically treated by amputation and adjuvant chemotherapy. Despite numerous efforts, the median survival time (MST) for dogs receiving a platinum compound, doxorubicin, or a combination of these remains at 8–12 months. Evidence from studies in mice suggests that gemcitabine has activity against OSA in vivo. Our preliminary work demonstrated that the addition of low-dosage (10 mM) gemcitabine to carboplatin resulted in synergistic inhibition of OSA cell viability in vitro.
Objective:
The purpose of the following study was to determine whether the addition of low-dosage (2 mg/kg) gemcitabine to carboplatin chemotherapy in dogs with OSA after amputation would improve MST over carboplatin monotherapy.
Animals:
Fifty dogs with histologically confirmed appendicular OSA.
Methods:
Dogs were treated prospectively with amputation and up to 4 dosages of carboplatin and gemcitabine in combination every 3 weeks.
Results:
The chemotherapeutic regimen was well tolerated with only 5 episodes of grade 3 or 4 hematologic toxicity. The median disease-free interval (DFI) was 203 days and the MST was 279 for all dogs in this study. The 1- and 2-year survival rates were 29.5 and 11.3%, respectively. Dogs with proximal humeral OSA had a shorter median DFI (P= .04) compared with dogs with OSA in other locations.
Conclusions and Clinical Importance:
These results are comparable to those reported for carboplatin monotherapy indicating that the addition of gemcitabine to carboplatin in dogs with appendicular OSA does not appear to improve outcome.
Osteosarcoma (OSA), the most common bone tumor in dogs, most often presents as a solitary tumor in the appendicular skeleton.1 Although fewer than 15% of dogs with appendicular OSA have evidence of gross metastases at the time of diagnosis, median survival times (MST) approach only 4 months after amputation, with affected patients dying because of progression of microscopic metastases present at diagnosis.2 MST are extended to 8–12 months if adjuvant chemotherapy with cisplatin, doxorubicin, carboplatin, or a combination of doxorubicin and a platinum compound is used.3–8 Nevertheless, >80% of dogs with OSA die of metastatic disease within 2 years. If substantial improvements in survival times are to occur, new therapeutic approaches are needed.
Gemcitabine (2′,2′-difluorodeoxycytidine) is a synthetic analog of the nucleoside analog cytosine arabinoside. Antitumor activity is achieved by inhibition of DNA replication and cell growth by incorporation of gemcitabine into replicating DNA and by blocking of repair mechanisms by masked DNA chain termination.9 Gemcitabine has been evaluated as a single agent and in combination with carboplatin in dogs with a variety of cancers. A Phase I study in dogs reported a maximal tolerated dosage of gemcitabine of 675 mg/m2 IV every 2 weeks.10 Gemcitabine administered weekly at 400 mg/m2 IV was evaluated in dogs with lymphoma resulting in no objective responses.11 In a later study, dogs with stages IV and V mammary carcinoma were treated postsurgery with weekly gemcitabine (800 mg/m2 IV).12 Although no clinically relevant toxicities were observed at this dosage, there was no difference in outcome in the dogs treated with surgery and gemcitabine compared with surgery alone. More recently, gemcitabine (2 mg/kg IV) followed by carboplatin (10 mg/kg IV) administered 4 hours later resulted in acceptable toxicity in dogs with carcinomas, but low response rates.13 It is important to note that while gemcitabine is typically administered to human patients over a 30-minute time infusion, administration over longer time courses (1–24 hours) has been shown to increase its efficacy in the treatment of solid tumors. Conversely, this increased infusion time is associated with higher rates of both nonhematologic and hemotologic (neutropenia, thrombocytopenia) toxicities (reviewed in Veltkamp et al14). These data indicate that the use of 20–30 minute gemcitabine infusion times in dogs may be associated with lower rates of biologic activity.
Carboplatin cytotoxicity is accomplished by DNA adduct formation and subsequent inhibition of protein synthesis and as such, gemcitabine and carboplatin are ideal candidates for use in combination given their different yet complementary mechanisms of action. This is supported by a variety of in vitro and in vivo studies. For example, gemcitabine and carboplatin were found to induce synergistic killing of canine OSA cells in vitro.15 The combination of gemcitabine and carboplatin demonstrated enhanced cytotoxicity in human nonsmall cell lung cancer lines and human endometrial carcinoma cell lines when coadministered in vitro.16,17 In human patients with platinum-resistant ovarian cancer receiving carboplatin followed by gemcitabine, a significant reduction in the repair of carboplatin-induced DNA cross-links was observed.18 Gemcitabine in combination with carboplatin is now the standard of care for human patients with advanced ovarian cancer that has recurred after the completion of platinum-based therapy.
Limited data exist regarding the role of gemcitabine for the treatment of soft tissue and bone sarcomas. Gemcitabine impaired the viability of human OSA cell lines in vitro and aerosolized gemcitabine inhibited the growth of human OSA xenografts and lung metastases in mice.19,20 Similarly, intranasal gemcitabine decreased the number of lung metastases in mouse models of OSA.21 In dogs with OSA metastatic to the lungs, aerosolized gemcitabine enhanced apoptosis and Fas expression.22
Our previous work investigating the activity of combined carboplatin and gemcitabine in canine OSA cell lines demonstrated synergism of carboplatin when combined with low-dosage (10 mM) gemcitabine.15 In this study, the IC50 for gemcitabine alone ranged from 5.7 to 15.3 μM for all OSA cell lines tested and the combination index indicated strong synergism at 1 μM gemcitabine/100 μM carboplatin. Based on the previous study evaluating the administration of carboplatin/gemcitabine to dogs, the recommended dosage of gemcitabine to be used with carboplatin was 2 mg/kg.13 Extrapolating from published reports describing gemcitabine pharmacokinetics in dogs,23–25 a dosage of 2 mg/kg would provide at least 3 hours of plasma drug concentration of 1 μM, a concentration at which synergistic drug activity was observed with carboplatin in the OSA cell lines. As such, the purpose of the clinical trial presented here was to expand upon our in vitro findings and evaluate the efficacy of combined carboplatin (300 mg/m2, standard dosage) and gemcitabine (2 mg/kg) in dogs with appendicular OSA after amputation.
Materials and Methods
Patients
Dogs with OSA were prospectively enrolled at The Ohio State University (OSU), the University of Wisconsin—Madison (UW—Madison), and the New England Veterinary Oncology Group (NEVOG) if they met the following criteria: weight >15 kg; histopathologically confirmed OSA of the appendicular skeleton with disease clinically confined to the leg; no evidence of distant metastasis to the lungs based on evaluation of 3-view thoracic radiographs at the time of presentation; amputation or limb-sparing procedure to remove the primary tumor; adequate organ function as indicated by CBC, serum biochemistry profile, and urinalysis; no chemotherapy or radiation therapy before amputation; and discontinuation of nonsteroidal anti-inflammatory drugs or corticosteroids 72 hours before initiation of chemotherapy and for the duration of the chemotherapy. The clinical protocol was approved by institutional Animal Care and Use Committees. All dogs enrolled were client owned, and written informed consent was obtained for each patient.
Subject Number
Assuming 80% power and 95% confidence interval (CI), 52 dogs were required to demonstrate a clinically significant increase in 1-year survival rate of 35–60% (Table 1) using the historical control groups of 155 dogs with appendicular OSA treated with amputation and carboplatin in a previously published study for comparison.3 The 1-year survival rate for dogs in this study was approximately 35%. An early withdrawal rate of 5% was assumed based on the expected tolerability of the protocol and historically high client compliance. Because the addition of gemcitabine was not expected to negatively influence survival outcomes, a 1-sided sample size was chosen for the power calculation. This analysis was performed by Stata v10.0.a

Chemotherapy Protocol
The objective was to begin chemotherapy treatment within 7–14 days after amputation. Carboplatin was administered at a dosage of 300 mg/m2 as an IV bolus before gemcitabine. The timing of this administration was determined based on published data indicating better inhibition of OSA cell viability when cells are exposed to carboplatin first, as well as a trend for improved survival times in human lung cancer patients when carboplatin is administered 4 hours before gemcitabine. Gemcitabine was diluted in 20–60 mL of 0.9% NaCl and administered at a dosage of 2 mg/kg as a 20-minute IV infusion 4 hours postcarboplatin administration. This dosage was chosen based on the previous use of gemcitabine with carboplatin13 as well as pharmacokinetic data indicating that 2 mg/kg will achieve a peak blood concentration of approximately 10 μM (2.6 μg/mL). Based on in vitro studies the combination index indicated strong synergism (combination index <0.3) at concentrations equal to 1/100 μM of gemcitabine/carboplatin in all OSA cell lines tested.15 This was achieved with exposures to gemcitabine and carboplatin of only 2 hours each. Therefore, our goal was to provide at least 2 hours of plasma drug concentration close to 1 μM (0.264 μg/mL). Based on the published studies after IV administration of gemcitabine at 3 mg/kg, the Cmax varies between 3 and 4 μg/mL and gemcitabine exhibits linear kinetics.24,25 The t1/2 in both studies was approximately 1.5–1.75 hours. Additionally, a plasma half-life of approximately 1.3–1.7 hours was found for dosages of 10, 30, and 60 mg/kg of gemcitabine, suggesting that a good approximation of half-life across all dosages is 1.5 hours.23 Therefore, given a dosage of 2 mg/kg, the Cmax would be predicted to be approximately 1–2 μg/mL with plasma concentrations of 0.5–1 μg/mL at 1.5 hours after administration and plasma concentrations of 0.25–0.5 μg/mL at 3 hours after administration. This would therefore provide at least 3 hours of plasma drug concentration of 1 μM. This chemotherapy regimen was administered every 21 days for a total of 4 cycles.
Patient Assessment during Treatment
Each patient was assessed once per week during the first cycle, then on day 1 of every cycle thereafter. A patient weight, physical examination, and CBC were performed during each visit. Three-view thoracic radiographs were obtained at initial evaluation, at the time of the 4th cycle of therapy, and every 3 months thereafter. Serum biochemical profiles were completed at enrollment and at the discretion of the attending clinician thereafter.
Treatment Delays and Dosage Reductions
Treatment delays were permitted by the attending clinician if the patient was systemically ill at the time of the chemotherapy appointment, if hematologic toxicity was observed at the time of the chemotherapy appointment (defined as an absolute neutrophil count <2,000/μL or a platelet count <100,000/μL), or if organ dysfunction was evident on routine laboratory tests. If treatment was delayed, the patient was reevaluated for treatment with a physical examination and the appropriate routine laboratory tests within 5–7 days. Dosage reductions were permitted at the discretion of the attending clinician for gastrointestinal toxicity, hematologic toxicity, or both.
Assessment of Toxicities
Toxicities were graded based on the grading system of adverse events (0–5) by the standard Veterinary Comparative Oncology Group—Common Terminology Criteria for Adverse Events criteria.26 Disease progression or clinical signs related to disease were not considered adverse events.
Data Analysis
Disease-free interval (DFI) was defined as the period from definitive primary tumor removal to the date the patient developed detectable metastasis. Survival was defined as the period from definitive primary tumor removal to the date the patient died or was euthanized. Every attempt was made to obtain a complete or partial necropsy at the time of death or euthanasia. DFI, overall survival (OS), and 1-year survival rate were calculated by the Kaplan-Meier product limit method. Dogs were censored from the DFI analysis if detectable recurrence or metastasis had not occurred at the time of data analysis, if death occurred before relapse because of causes not related to the OSA, or if they were lost to follow-up. Dogs were censored from the survival analysis if they were alive at the time of data analysis, if they did not die from OSA, or if they were lost to follow-up. Survival data were compared with historical data on dogs with OSA treated with amputation and single-agent carboplatin.3 Identification of prognostic factors influencing DFI and OS by univariate analysis by the log-rank (Mantel-Cox) test was performed on the following variables: tumor location, age (≥5 years), sex, weight (≥25 kg), breed, total serum alkaline phosphatase activity (normal versus increased), and time to initiation of chemotherapy (>14 days postamputation). Lastly, an analysis by the log-rank (Mantel-Cox) test was performed comparing dogs treated in our population with raw disease-free and OS data generated in a previously published historical group of dogs with OSA treated with single-agent carboplatin after amputation.3
Results
Patient Characteristics
Fifty-two dogs with appendicular OSA met the inclusion criteria and were enrolled prospectively into this clinical trial between February 25, 2008, and May 19, 2009. Two dogs were excluded from data analysis; 1 did not return for chemotherapy postamputation and 1 was excluded because the OSA was located in the calcaneus. Twenty-four dogs were enrolled at OSU, 15 at NEVOG, and 11 at UW—Madison. The final assessment for all dogs enrolled in this study was performed on July 5, 2010. There were 12 mixed-breed dogs and 38 purebred dogs. The breeds represented included Labrador Retriever (n = 9), Great Dane (n = 3), German Shepherd (n = 3), Rottweiler (n = 3), Golden Retriever (n = 2), Great Pyrenees (n = 2), Irish Setter (n = 2), Irish Wolfhound (n = 2), and 1 of each of the following breeds: Doberman Pinscher, Hungarian Vizsla, German Shorthair Pointer, Boxer, Siberian Husky, Samoyed, Saint Bernard, and English Mastiff. There were 27 female (25 spayed) and 23 male (22 castrated) dogs. The mean and median ages were 8.1 and 8.0 years, respectively (range, 2–15 years). The mean and median weights were 36.0 and 33.5 kg, respectively (range, 19.8–72.6 kg).
Tumor Characteristics
Tumors were located in the distal radius (n = 15), proximal humerus (n = 14), distal femur (n = 8), distal tibia (n = 6), proximal tibia (n = 3), proximal femur (n = 2), and 1 each in the proximal radius and distal ulna. Eight dogs had abnormally total serum alkaline phosphatase activity at the time of diagnosis with mean and median values of 339 and 263 IU/L, respectively (range, 125–829 IU/L).
Chemotherapy Protocol
The median time from amputation to the initiation of chemotherapy was 14 days (range, 8–41 days). Twenty-one dogs received their 1st chemotherapy treatment >14 days (median, 19 days) postamputation because of infection at the amputation site (n = 1), dehiscence of the amputation site (n = 1), and, in 19 dogs, the reason for the delay in initiation of chemotherapy was not reported. Thirty-seven dogs (74%) received all 4 chemotherapy treatments, 9 dogs received 3 treatments and 1 dog received 2 treatments because of development of metastatic disease before subsequent treatments, and 3 dogs received only 1 treatment (1 dog died acutely after experiencing severe vomiting and diarrhea after the 1st chemotherapy treatment and 2 dogs developed metastatic disease before the 2nd treatment).
Clinical Toxicities
The median dosage of carboplatin for all treatment cycles was 300 mg/m2 (range, 240–316 mg/m2) and the median dosage of gemcitabine for all treatment cycles was 2 mg/kg (range, 1.81–2.10 mg/kg). Fifteen dosage reductions of carboplatin were made in 6 dogs at the time of the 2nd (n = 4) or 3rd (n = 2) treatment cycle for the following reasons: grade 3 neutropenia (n = 2), grade 4 neutropenia and grade 3 thrombocytopenia (n = 1), and grade 2 lethargy and grade 2 anorexia (n = 1). In 2 dogs, a reason was not given for the dosage reduction of carboplatin (range, 280–287 mg/m2 in these 2 dogs). In the dogs that received dosage reductions, the median dosage of carboplatin was 254 mg/m2 (range, 240–287 mg/m2). The dosage of gemcitabine was not reduced in any of the study patients. Six treatment delays were made in 6 dogs because of neutropenia (grade 1, n = 3; grade 2, n = 2; grade 4, n = 1), thrombocytopenia (grade 3, n = 1), or both. The average treatment delay was 4 days (range, 2–9 days).
Adverse events related to chemotherapy generally were mild and self-limiting. There were 23 episodes of hematologic toxicity in total. These included 17 episodes of neutropenia in 9 dogs (grade 1, n = 6; grade 2, n = 7; grade 3, n = 3; and grade 4, n = 1). The median neutrophil counts 1, 2, and 3 weeks after the 1st treatment cycle were 3.2 × 103/L (range, 1.32–7.53 × 103/L), 3.28 × 103/L (range, 0.71–12.71 × 103/L), and 5.19 × 103/L (range, 1.51–16.25 × 103/L), respectively. Thrombocytopenia occurred in 6 dogs (grade 2, n = 5; grade 3, n = 1). The median platelet counts 1, 2, and 3 weeks after the 1st treatment cycle were 235 × 103/L (range, 55–792 × 103/L), 198 × 103/L (range, 50–720 × 103/L), and 400 × 103/L (range, 166–856 × 103/L).
There were 17 documented episodes of grade 1 or 2 gastrointestinal toxicity in 13 dogs including anorexia (grade 1, n = 7; grade 2, n = 4), nausea (grade 1, n = 1), vomiting (grade 1, n = 1; grade 2, n = 1), and diarrhea (grade 1, n = 2; grade 2, n = 1). There were no grade 3 or 4 gastrointestinal toxicities reported. One dog experienced several episodes of vomiting, hemorrhagic diarrhea, and died acutely 4 days after the 1st carboplatin and gemcitabine treatment. No clinically relevant abnormalities were observed in this dog at necropsy including no obvious gross or histopathologic lesions in the gastrointestinal tract, and therefore the cause of death could not be determined. There were 2 episodes of grade 1 hyperbilirubinemia in 2 dogs, which resolved without treatment and did not occur after subsequent treatments with carboplatin and gemcitabine.
Additional Treatments
Additional treatments were permitted at the time of development of metastatic disease. These were instituted in only 7 dogs and included a combination of toceranib phosphate (Palladia), cyclophosphamide and piroxicam (n = 2), doxorubicin alone (n = 3), Cyberknife radiation therapy in combination with pamidronate and doxorubicin (n = 1), or doxycycline and carprofen as part of a metronomic chemotherapy protocol (n = 1).
Clinical Outcome
The overall median DFI was 203 days (95% CI; 164–245 days) with a range of 22–469 days (Fig 1). Seven dogs were censored in the DFI data analysis because recurrence or metastasis had not occurred before the end of the study period in 6 dogs and 1 patient died of acute leukemia before relapse. The median duration of follow-up was 518 days for the 7 patients censored in the DFI analysis, indicating an adequate follow-up time. The overall MST was 279 days (95% CI; 201–340 days) with a range of 22–719 days (Fig 2). The 1- and 2-year survival rates were 29.5 and 11.3%, respectively. There were 10 censored subjects in the survival analysis for the following reasons: 9 were alive at the time of data analysis and 1 died of acute leukemia 143 days after the OSA diagnosis. The median DFI and OS in our study population were not statistically different from the previously published historical control population (Phillips et al3) treated with carboplatin monotherapy (P= .10 and .06, respectively, Figs 1 and 2). In that population, the median DFI was 256 days (95% CI; 181–334 days) and the median OS was 307 days (95% CI; 250–406 days).
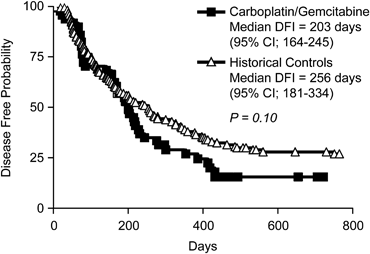
Figure 1. Kaplan-Meier disease-free interval (DFI) curves comparing dogs treated with carboplatin/gemcitabine to historical controls treated with carboplatin chemotherapy.

Figure 2. Kaplan-Meier overall survival (OS) curves comparing dogs treated with carboplatin/gemcitabine combination to historical controls treated with carboplatin chemotherapy.
Of the 41 dogs with documented metastatic disease, 28 (56%) developed pulmonary metastases, 17 (34%) developed metastases to bone or second primary lesions in skeletal sites, 3 (6%) developed metastasis to the skin, 2 (4%) developed intraabdominal metastases, and 1 developed metastasis to the regional lymph node. Ten (24.4%) of the 41 dogs with metastatic disease were diagnosed with multifocal metastatic lesions. Dogs with pulmonary and bone lesions most often were diagnosed with metastatic disease based on radiographic appearance with or without concurrent clinical signs of disease, (ie, pain, lameness, or both associated with the bone lesion). Dogs with skin, lymph node, or intraabdominal metastases were diagnosed by cytology (n = 4) or histopathology (n = 2).
Prognostic Factors
Given the fact that several dogs had delay of chemotherapy administration beyond day 14, this was evaluated as a prognostic indicator. Median OS for dogs that received treatment >14 days postamputation was 279 days (n = 29) compared with 346 days (n = 21) for the dogs that received treatment 7–14 days postamputation, although this was not significant (P= .5334). Of the other variables analyzed, only proximal humeral location was associated with a significantly shorter DFI (Fig 3). Dogs with proximal humeral OSA (n = 14) had a median DFI of 121 days (95% CI; 75–232 days) compared with a median DFI of 220 days (95% CI; 172–299 days) for dogs with appendicular OSA in other locations (P= .04).

Figure 3. Kaplan-Meier disease-free interval (DFI) curves for dogs with osteosarcoma (OSA) in the proximal humerus treated with amputation followed by carboplatin and gemcitabine chemotherapy versus dogs with OSA in other locations that underwent the same treatment (P = .04).
Discussion
Because we had demonstrated previously that the combination of carboplatin and gemcitabine exhibited synergistic antitumor activity in vitro against canine OSA cell lines, the purpose of this clinical trial was to evaluate the efficacy of combined carboplatin/gemcitabine treatment in vivo in dogs with appendicular OSA after amputation. The patient characteristics in this prospective clinical trial were similar to those reported in 2 previous studies that evaluated carboplatin monotherapy for the treatment of dogs with OSA.3,4 The median DFI in our study population was 203 days, which is similar to the previously reported median DFI of 257 days for dogs that received single-agent carboplatin postamputation in a prospective clinical trial (n = 48, Bergman et al4) and not statistically different than the 256-day median DFI in dogs that received single-agent carboplatin either before or after amputation in a large retrospective study (n = 157, Phillips et al3). The MST and 1-year survival rate in dogs that received the carboplatin/gemcitabine regimen postamputation was 279 days and 29.5%, respectively, which is also relatively comparable to the 307 days and 36.8% 1-year survival rate reported by Phillips et al.3 In a prospective study of 303 dogs that received doxorubicin chemotherapy postamputation with or without an inhibitor of matrix metalloproteinases (BAY 12-9566), the MST was 8 months regardless of whether patients received BAY 12-9566 or placebo.8 Therefore, the addition of gemcitabine to standard carboplatin chemotherapy did not improve either DFI or MST over carboplatin alone or doxorubicin alone in dogs with microscopic metastatic OSA after amputation.
Of the dogs that had developed metastatic disease in this study, 56% had pulmonary metastases, 34% had metastases to skeletal sites or secondary primary bone lesions, 6% had metastasis to the skin, and 4% had intraabdominal metastases. The percentage of dogs that had pulmonary and intraabdominal metastases was lower in the current study when compared with that reported by Bergman and colleagues in which 91% of dogs that died had necropsies performed. Although 24% of dogs diagnosed with metastatic disease had multifocal metastatic lesions in the current study, it is likely that this percentage underrepresents the extent of metastatic disease because necropsies were not routinely obtained.
We evaluated several factors that have been reported previously to negatively influence DFI, survival, or both, including tumor location, age, sex, weight, breed, total serum alkaline phosphatase activity (normal versus increased), and time to initiation of chemotherapy (≥14 days postamputation). The only negative prognostic factor identified in our study was proximal humeral location on DFI. The proximal humeral location was first reported to be a negative prognostic factor by Bergman et al4 who reported a median DFI of 139 days in 9 dogs with proximal humeral OSA. This negative prognostic factor on DFI was again reported by Phillips et al3 (median DFI 177 days in 40 dogs with proximal humeral OSA). In the current study, median DFI in dogs with OSA in the proximal humeral location was 121 days compared with 220 days in dogs with OSA in other appendicular locations, and 5/12 dogs (41%) that had developed gross metastasis by the time of the 4th treatment cycle had OSA in the proximal humerus.
Complications associated with chemotherapy generally were mild and reversible; however, 1 dog (1/50, 2%) acutely died at home after experiencing several episodes of vomiting and hemorrhagic diarrhea after the 1st carboplatin/gemcitabine treatment. Although the exact cause of death could not be determined on necropsy, it is suspected that this patient died of gastrointestinal complications from chemotherapy. This is consistent with previous studies of carboplatin monotherapy for the treatment of appendicular OSA, in which 6/205 treated dogs (2.9%) died after chemotherapy-induced toxicities.3,4
There are limitations of this prospective clinical trial that should be addressed. The current study did not use a contemporaneous control group of dogs that received single-agent carboplatin. Amputation and administration of single-agent carboplatin has been used extensively in the treatment of appendicular OSA and is one of the standards of care for dogs with this disease. We powered our study and compared our results statistically to historical information published previously for 155 dogs with OSA that underwent amputation and received single-agent carboplatin.3 Although the patient population was similar to that reported previously, it is difficult to make direct comparisons among nonrandomized groups of dogs with OSA. That being said, neither the DFI nor the OS differed significantly from that in the historical population, suggesting that the addition of gemcitabine to the therapeutic regimen did not improve outcome in this disease setting.
A 2nd limitation is that the maximal tolerated dosage of gemcitabine in combination with carboplatin may not have been used, as there was only 1 nonhematologic serious adverse event and very few grades 3 and 4 hematologic toxicities, none of which induced clinical toxicities (eg, febrile neutropenia). The chemotherapy protocol in this study was chosen based on that often used in human patients,27–29 a previous clinical trial evaluating carboplatin/gemcitabine in dogs with carcincomas,13 and pharmacokinetic data indicating that gemcitabine at 2 mg/kg will provide at least 3 hours of plasma drug concentration of 1 μM (the concentration necessary to produce synergistic effects in combination with carboplatin in canine OSA cell lines).23–25 Given the minimal and acceptable toxicity of the treatment protocol used, higher dosages of gemcitabine may have been tolerable and potentially resulted in greater clinical activity. As such, a dosage escalation study that increases the dosage of gemcitabine while keeping the dosage of carboplatin constant may be appropriate to determine if higher dosages of gemcitabine are tolerated and could be used in future combination studies.
A 3rd possible limitation of this trial involves the sequence of drug administration. In this study, carboplatin was administered 4 hours before gemcitabine based on in vitro data indicating better biologic activity when OSA cells were exposed to carboplatin before gemcitabine compared with the reverse sequence of administration.15 Although the data regarding sequence of administration is controversial in human oncology, there is some evidence suggesting that certain types of cancers may respond preferentially to specific sequencing of the 2 drugs. In 1 study, carboplatin and gemcitabine treatment were synergistic in a human lung carcinoma cell line only when carboplatin was administered before gemcitabine. These data were supported by the fact that the administration of carboplatin 4 hours before gemcitabine was associated with higher response rates and longer survival times in clinical patients with non-small-cell lung cancer when compared with patients who were treated with drug in the reverse order, although this did not reach statistical significance.27 Conversely, another study showed that the sequence of administration of carboplatin and gemcitabine did not affect toxicity, pharmacodynamics, the maximal tolerated dosage, or response rates in patients with non-small-cell lung cancer.28 In our preclinical work, we demonstrated that that addition of gemcitabine to carboplatin resulted in synergistic inhibition of OSA cell viability in vitro and found that growth inhibition was greater in 3/4 canine OSA cell lines when they were treated with carboplatin 4 hours before gemcitabine rather than with gemcitabine 4 hours before carboplatin.15
In summary, although the combination of carboplatin/gemcitabine was well tolerated in dogs after amputation for appendicular OSA, DFI and median OS were not improved over that reported for dogs receiving single-agent carboplatin. Recently there have been substantial advances in palliative treatment options for dogs with OSA; however, there have been no clinically relevant improvements in survival times for dogs that undergo amputation and adjuvant chemotherapy for over a decade. This study further demonstrates the need for novel therapeutic approaches beyond the use of standard chemotherapeutics aimed at the treatment of micrometastatic lesions in this patient population.
Acknowledgments
The authors thank members of the Clinical Trials staff at The Ohio State University, University of Wisconsin—Madison, and New England Veterinary Oncology Group, as well as members of the medical oncology services at these institutions, for their assistance with this clinical trial.
References
-
1
, Withrow and MacEwen's Small Animal Clinical Oncology, 4th ed. St. Louis, MO: Saunders Elsevier; 2007:540–573.
-
2
Spodnick GJ, Berg J, Rand WM, et al. Prognosis for dogs with appendicular osteosarcoma treated by amputation alone: 162 cases (1978–1988). J Am Vet Med Assoc 1992;200:995–999.
-
3
Phillips B, Powers BE, Dernell WS, et al. Use of single-agent carboplatin as adjuvant or neoadjuvant therapy in conjunction with amputation for appendicular osteosarcoma in dogs. J Am Anim Hosp Assoc 2009;45:33–38.
-
4
Bergman PJ, MacEwen EG, Kurzman ID, et al. Amputation and carboplatin for treatment of dogs with osteosarcoma: 48 cases (1991 to 1993). J Vet Intern Med 1996;10:76–81.
- Web of Science® Times Cited: 111
-
5
Chun R, Garrett LD, Henry C, et al. Toxicity and efficacy of cisplatin and doxorubicin combination chemotherapy for the treatment of canine osteosarcoma. J Am Anim Hosp Assoc 2005;41:382–387.
-
6
Kent MS, Strom A, London CA, et al. Alternating carboplatin and doxorubicin as adjunctive chemotherapy to amputation or limb-sparing surgery in the treatment of appendicular osteosarcoma in dogs. J Vet Intern Med 2004;18:540–544.Direct Link:
-
7
Bailey D, Erb H, Williams L, et al. Carboplatin and doxorubicin combination chemotherapy for the treatment of appendicular osteosarcoma in the dog. J Vet Intern Med 2003;17:199–205.Direct Link:
-
8
Moore AS, Dernell WS, Ogilvie GK, et al. Doxorubicin and BAY 12-9566 for the treatment of osteosarcoma in dogs: A randomized, double-blind, placebo-controlled study. J Vet Intern Med 2007;21:783–790.
-
9
Chabner BA, Longo DL. Cancer Chemotherapy and Biotherapy: Principles and Practice, 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
-
10
Kosarek CE, Kisseberth WC, Gallant SL, et al. Clinical evaluation of gemcitabine in dogs with spontaneously occurring malignancies. J Vet Intern Med 2005;19:81–86.Direct Link:
-
11
Turner AI, Hahn KA, Rusk A, et al. Single agent gemcitabine chemotherapy in dogs with spontaneously occurring lymphoma. J Vet Intern Med 2006;20:1384–1388.Direct Link:
-
12
Marconato L, Lorenzo RM, Abramo F, et al. Adjuvant gemcitabine after surgical removal of aggressive malignant mammary tumours in dogs. Vet Comp Oncol 2008;6:90–101.Direct Link:
-
13
Dominguez PA, Dervisis NG, Cadile CD, et al. Combined gemcitabine and carboplatin therapy for carcinomas in dogs. J Vet Intern Med 2009;23:130–137.Direct Link:
-
14
Veltkamp SA, Beijnen JH, Schellens JH. Prolonged versus standard gemcitabine infusion: Translation of molecular pharmacology to new treatment strategy. Oncologist 2008;13:261–276.
-
15
McMahon MB, Bear MD, Kulp SK, et al. Biological activity of gemcitabine against canine osteosarcoma cell lines in vitro. Am J Vet Res 2010;71:799–808.
-
16
Edelman MJ, Quam H, Mullins B. Interactions of gemcitabine, carboplatin and paclitaxel in molecularly defined non-small-cell lung cancer cell lines. Cancer Chemother Pharmacol 2001;48:141–144.
-
17
Smith JA, Brown J, Martin MC, et al. An in vitro study of the inhibitory activity of gemcitabine and platinum agents in human endometrial carcinoma cell lines. Gynecol Oncol 2004;92:314–319.
-
18
Ledermann JA, Gabra H, Jayson GC, et al. Inhibition of carboplatin-induced DNA interstrand cross-link repair by gemcitabine in patients receiving these drugs for platinum-resistant ovarian cancer. Clin Cancer Res 2010;16:4899–4905.
-
19
Koshkina NV, Kleinerman ES. Aerosol gemcitabine inhibits the growth of primary osteosarcoma and osteosarcoma lung metastases. Int J Cancer 2005;116:458–463.
-
20
Ando T, Ichikawa J, Okamoto A, et al. Gemcitabine inhibits viability, growth, and metastasis of osteosarcoma cell lines. J Orthop Res 2005;23:964–969.
-
21
Jia SF, Worth LL, Turan M, et al. Eradication of osteosarcoma lung metastasis using intranasal gemcitabine. Anticancer Drugs 2002;13:155–161.
-
22
Rodriguez CO, Crabbs TA, Wilson DW, et al. Aerosol gemcitabine: Preclinical safety and in vivo antitumor activity in osteosarcoma-bearing dogs. J Aerosol Med Pulm Drug Deliv 2010;23:197–206.
-
23
Freise KJ, Martin-Jimenez T. Pharmacokinetics of gemcitabine and its primary metabolite in dogs after intravenous infusion. J Vet Pharmacol Ther 2006;29:147–152.Direct Link:
-
24
Freise KJ, Martin-Jimenez T. Pharmacokinetics of gemcitabine and its primary metabolite in dogs after intravenous bolus dosing and its in vitro pharmacodynamics. J Vet Pharmacol Ther 2006;29:137–145.Direct Link:
-
25
Shipley LA, Brown TJ, Cornpropst JD, et al. Metabolism and disposition of gemcitabine, and oncolytic deoxycytidine analog, in mice, rats, and dogs. Drug Metab Dispos 1992;20:849–855.
-
26
Group VCO. Common terminology criteria for adverse events (VCOG—CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.0. Vet Comp Oncol 2004;2:195–213.Direct Link:
-
27
Bajetta E, Stani SC, De Candis D, et al. Preclinical and clinical evaluation of four gemcitabine plus carboplatin schedules as front-line treatment for stage IV non-small-cell lung cancer. Ann Oncol 2003;14:242–247.
-
28
Langer CJ, Calvert P, Ozols RF. Gemcitabine and carboplatin in combination: Phase I and phase II studies. Semin Oncol 1998;25:51–54.
-
29
Pfisterer J, Plante M, Vergote I, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: An intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol 2006;24:4699–4707.
Share this article / Teilen Sie diesen Artikel
Diese Webseite wurde mit Jimdo erstellt! Jetzt kostenlos registrieren auf https://de.jimdo.com








