
Article added / Artikel hinzugefügt 01.10.2021
Generally Articles and Discussions about Osteosarcoma in Dogs
→ Evaluations of phylogenetic proximity in a group of 67 dogs with
osteosarcoma: a pilot study
Article added / Artikel hinzugefügt 01.10.2021
Generally Articles and Discussions about Osteosarcoma in Dogs
→ Canine Periosteal Osteosarcoma
Images added / Abbildungen hinzugefügt 02.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Pulmonary vessels
New subcategory added / Neue Unterkategorie hinzugefügt 02.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Pulmonary vessels
Images added / Abbildungen hinzugefügt 01.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Heart valvular diseases



Assessment of Diastolic Function by Doppler Echocardiography in Normal Doberman Pinschers and Doberman Pinschers with Dilated Cardiomyopathy

O'Sullivan, M. L., O'Grady, M. R. and Minors, S. L. (2007), Assessment of Diastolic Function by Doppler Echocardiography in Normal Doberman Pinschers and Doberman Pinschers with Dilated Cardiomyopathy.
Journal of Veterinary Internal Medicine, 21: 81–91.
doi: 10.1111/j.1939-1676.2007.tb02932.x

Background:
Assessment of diastolic function in patients with dilated cardiomyopathy (DCM) has the potential to add
valuable information regarding hemodynamics, disease severity, and prognosis. The purpose of this study was to determine transmitral flow (TMF), isovolumic relaxation time (IVRT), pulmonary
venous flow (PVF), flow propagation velocity (Vp), and mitral annular velocities by tissue Doppler in Doberman Pinschers with and without DCM. Hypothesis:
It was anticipated that normal and DCM Dobermans would differ with respect to these parameters, and that
associations with time to congestive heart failure (CHF) or death would be found.
Animals:
Thirty client-owned Doberman Pinschers (10 each of normal, occult DCM, and overt DCM) were studied.
Methods:
Each dog underwent echocardiography with or without thoracic radiography (to confirm CHF) for classification as normal or DCM-affected, followed by collection of echocardiographic diastolic
parameters.
Results:
The group with occult DCM exhibited features of pseudonormal TMF, reduced systolic to diastolic PVF ratio, and
reduced Vp. Shorter early TMF deceleration time (DTE) was associated with shorter time to CHF or sudden death. The group with overt DCM exhibited restrictive
TMF, blunted systolic PVF, and reduced early and late diastolic mitral annular velocities.
Conclusions and Clinical Importance:
Doberman Pinschers showed evidence of moderate and severe diastolic dysf unction in occult and overt DCM, respectively. Short DTE may be a useful predictor of onset of CHF or sudden death.
Key words:
Canine; Flow propagation velocity; Pulmonary venous flow; Tissue Doppler imaging; Transmitral flow.
Idiopathic dilated cardiomyopathy (DCM) is a primary myocardial disease characterized by systolic dysfunction and
secondary eccentric hypertrophy of 1 or both ventricles in the absence of a definable cause, likely representing a
common expression of myocardial damage produced by a variety of unestablished myo-cardial insults or
genetic derangements.1 Doberman Pinschers present a unique opportunity for investigation of this disease due to the high incidence
(up to 50%) experienced by this breed.2 The natural history of DCM is characterized by an occult phase during which there is echocardiographic or
electrocardiographic (eg, ventric-
ular tachyarrhythmias) evidence of cardiomyopathy but no clinical signs of disease. Progression of the disease
leads ultimately to the overt phase during which dogs experience signs of congestive heart failure (CHF)
or
sudden death (SD). Diastolic dysfunction, defined as increased resistance to filling of 1 or both
ventricles,
likely plays an important role in the transition between occult and overt disease, promoting pulmonary congestion and exacerbating arrhythmias.3 Diastolic dysfunction also
may become manifest very early in the disease process, even before clinically detectable systolic dysfunction can be identified by standard echocardiographic
measures.4
Characterization of diastolic function thus may be useful in the assessment of DCM patients, potentially yielding valuable
information about hemodynamics, disease severity, therapeutic response, and prognosis.
Doppler echocardiography combining transmitral flow (TMF), isovolumic relaxation time (IVRT), and pulmonary venous flow
(PVF) is accepted as a reliable, reproducible, and practical means of assessing diastolic function in humans.5–9 Many of these indices correlate
closely with symptom status and have utility in predicting prognosis and
therapeutic response.5,10–14 Noninvasive assessment of diastolic function may be enhanced, however, by the measurement of left ventricular (LV)
flow propagation velocity (Vp) with color M-mode echocardiography and mitral annular motion by tissue Doppler imaging (TDI), because these indices are
cited as being preload independent unlike those above.7,15–17 A small
number of veterinary studies have
described TMF and PVF, 18,19 and myocardial motion by TDI 20 in normal dogs. Limited studies involving canine
DCM patients have evaluated TMF or PVF, 21–23 and the utility of TDI has been demonstrated in the
early
diagnosis of puppies affected with Golden Retriever Muscular Dystrophy (GRMD).4 To the authors’ knowledge, a comprehensive noninvasive
assessment of diastolic function incorporating all of the aforementioned modalities in naturally occurring canine DCM has not been reported.
The purpose of this study was to describe diastolic function by means of a combination of
echocardio-graphic indices (TMF, IVRT, PVF, Vp, and TDI) in Doberman Pinschers with occult DCM and overt DCM in comparison with normal Doberman
Pinschers, and to investigate the presence of any associations between
these indices and prognosis.
Materials and Methods
The study protocol was approved by the Animal Care Committee of the
University of Guelph, and informed consent was obtained from all owners.
Patients
All patients were client-owned Doberman Pinschers 20 months of age examined between March
2001 and May 2002 at the Small Animal Teaching Hospital of the Ontario Veterinary College, University of
Guelph. Each dog underwent a physical examination, 9-lead ECG, and echocardiography. Echocardiography for screening was performed by
an experienced echocardiographer (MRO) on dogs manually restrained in right
lateral
recumbency with an echocardiographic system equipped with either a 2–4 or 3–5 MHz transducer. a A2 - dimensional guided
M-mode examination from the right parasternal long-axis view was used to measure LV internal dimension in diastole (LVIDd) and in systole
(LVIDs), and fractional shortening (FS) was calculated.
The average of 3 measurements, with reacquisition of the image for each, was used. Dogs were included in the normal group (NL) if they were free of clinical
signs of cardiac disease and if all of the following criteria were met: LVIDd,42.7 mm for males or ,40.9 mm for
females; LVIDs,34.7 mm for males or,33.1 mm for females; and no ventricular premature beats during the ECG and echocardiogram. b,c
Dogs were included in the occult DCM group (OccDCM) if they were free of clinical signs of cardiac
disease and if the following criteria for LV enlargement were met: LVIDd .49 mm or LVIDs.42 mm.d Dogs were included in the overt DCM group (OvDCM) if all
of the following criteria were met: LV enlargement as above for the occult DCM group; FS#16%;
respiratory clinical signs (any of cough, wheeze, dyspnea, orthopnea); and radiographic evidence of
pulmonary edema. Exclusion criteria included evidence of mitral valve disease (ie, marked mitral regurgitation,
abnormal mitral valve morphology, and exuberant septal motion), any cardiac disease other than
DCM, or the presence of atrial fibrillation. The medications received by and the diets fed to the
dogs were not standardized. All OccDCM dogs were receiving angiotensin-converting enzyme inhibitors (ACEI), and 2 were receiving
beta-blockers at the time of examination. All OvDCM dogs were receiving ACEI and
furosemide; and 3 were also on sotalol; 1 was also on spironolactone; and 1
had received a dobutamine infusion within
the previous 48 hours.
Echocardiographic Measurements of Diastolic Function
All echocardiographic examinations of diastolic function were performed by 1 experienced echocardio-grapher (MLO). Patients
were restrained manually in left lateral recumbency and the same echocardiographic system equipped with a 2–4 MHz transducer was used.a All
indices were acquired from the apical 4-chamber
view, except IVRT, which was obtained from the apical 5-chamber view. Continuous wave (CW) and pulsed wave (PW) Doppler and color M-mode recordings were made at a sweep speed of 100 mm/s. Three
observations, with reacquisition of the image, were obtained for each parameter, and the average was used in data analysis. R-wave to R-wave intervals, both preceding and including the cycles
used for measurement, were recorded to calculate instantaneous heart rate. All measurements were performed at
end-expiration with the aid of a respiratory monitor. All TMF, IVRT, and PVF measurements were performed as described by Rakowski et al 6 and with the aid of a
technical guide. 24 Color M-mode LV flow propagation and TDI measurements were performed according to
Garcia et al.15
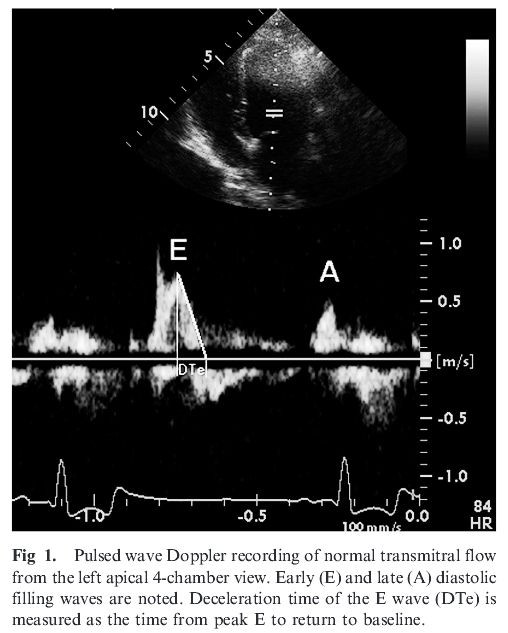
In brief, TMF was recorded with PW spectral Doppler with a 2-mm-long sample volume placed at the tips of the open mitral valve
leaflets. Color Doppler was used to help align the cursor parallel to mitral inflow.
Measurements included peak early (E) and late (A) diastolic filling velocities, the duration of the
late-filling wave (A dur), and deceleration time of the early-filling wave (DTE) (Fig 1). The ratio E : A was calculated. IVRT was
measured with CW spectral Doppler as the time from the aortic valve closure artifact to the mitral valve opening artifact or to the
onset of mitral inflow if the mitral valve opening artifact was not obtained.
PVF was recorded by PW spectral Doppler interrogation of the left caudal lobar pulmonary vein (PV). Color
Doppler was optimized for low velocity flow. The PW sample volume was placed about 1 cm into the PV. Sample volume length
initially was set at 1–2 mm but was changed to 3–4 mm if the spectral Doppler signal was poor. Measurements included peak systolic (S), diastolic (D), and atrial reversal (AR) flow velocities
(Fig 2), and duration
of the AR wave (AR dur). The ratio S : D and the ratio of TMF A duration to PVF AR duration were calculated.
Vp was determined from a color M-mode recording of LV inflow. The color velocity scale was adjusted by shifting the baseline to optimize color aliasing of the mitral inflow signal (between 35 and 55 cm/s). Vp was measured as the slope of the first aliasing isovelocity line of early diastolic flow, starting at the level of the mitral annulus and extending as far as possible toward the LV apex (at least 2.5 cm) (Fig 3).
Mitral annular motion was measured from the lateral (free wall) side with PW TDI. Color TDI was used to aid in sample volume placement, and the cursor was aligned as parallel as possible to the
longitudinal axis of LV wall motion. A sample volume length of 5 mm was used, and Doppler gain was minimized. Measurements included
peak early diastolic (Em), late diastolic (Am), and systolic (Sm) mitral annular velocities (Fig 4), with calculation of Em:Am
ratio.

Follow-up
For the OccDCM group, time to CHF or SD was recorded as the number of days from enrollment (day of data collection) to the onset of respiratory signs and need
for diuretics, or to SD. Dogs experiencing noncardiac death before one of the above endpoints or those still occult at the end of the study follow-up period (March
2003) were right censored. For the OvDCM group, survival time was calculated as the number of days from enrollment to CHF death, SD, or euthanasia because of CHF. Dogs experiencing noncardiac
death before one of the above endpoints were right censored.
Statistical Analysis
Statistical analyses were performed by computer-based statistical software.e Tests of normality (Shapiro-Wilk and Kolmogorov-Smirnov tests, significance level P. .1) were applied and verified by
examining residual plots. Log transformations were performed if residuals were not normally distributed. Differences among groups in
baseline characteristics were assessed by analysis of variance (ANOVA) and the Least squares difference (LSD) test for multiple
comparisons. Differences among groups in diastolic indices were evaluated by analysis of
covariance (ANOVA of values adjusted for regression on an independent variable),25 with the following potential covariates included: sex, age, body weight (BW),
and heart rate (HR). Interactions between covariates,
between covariates and the group effect, and quadratic terms for age and BW also were included. Least squares means (LS means), which incorporate
and thus account for the effects of significant covariates (essentially adjusting the
means to account for differences between groups in significant covariates), were calculated
for each variable based on the respective model.25 For example, if age was a
significant covariate for E : A ratio, then differences between groups in E : A ratio may have been the result of differences in age, therefore E : A ratio must be
adjusted for age to make the group means comparable. Tests of multiple comparisons then
were applied (LSD test for comparison of 3 groups, and Tukey’s test for comparison of 6 groups when sex was
a significant covariate). Bivariate Cox proportional-hazards regression analysis was used to determine if any individual variables were significantly
associated with time to endpoint in the OccDCM or OvDCM groups. The variables examined included age, BW, sex, LVIDd, LVIDs, FS, and all echocardiographic
diastolic function indices. A proportional hazard ratio (risk ratio, RR) with 95% confidence interval (CI) was
calculated for each variable. Significance was defined as P, .05.
Results
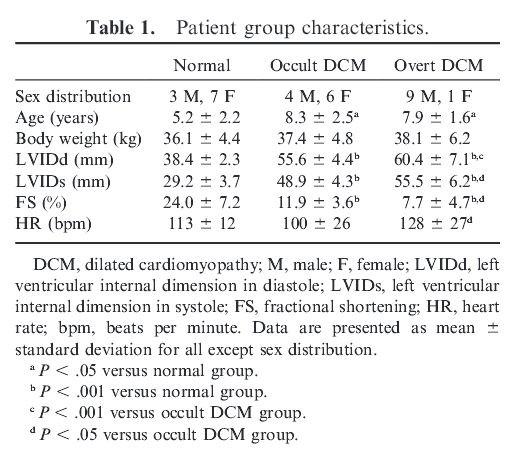
A total of 30 Doberman Pinschers were included in the study, with 10 dogs in each of the 3 groups. Sex, age,
BW, LV dimensions, and initial HR for each of the 3 groups are summarized in Table
1, with significant
differences among groups noted. Statistical description of the diastolic function data in the 3 patient groups is
presented in Table 2. Table 3 summarizes the results of the analysis of covariance, including significant covari-
ates, LS means with 95% CI, and results of the multiple group comparisons. The LS means for variables with
continuous covariates are those calculated with the average values of the continuous
covariates (age, BW,
HR). Separate LS means are reported for males and females if sex was a
significant covariate for that
particular variable.
TMF with separate E and A waves was obtained in all 30 dogs. Good quality PVF was obtained in 10/10 NL, 9/10
OccDCM, and only 5/10 OvDCM dogs. Vp was obtained in all NL and OccDCM dogs, but in only 9/10 OvDCM dogs because a color
M-mode signal of adequate depth could not be obtained in 1 dog. Mitral
annular velocities by TDI were obtained in all NL and OccDCM dogs, but in only 8/10 OvDCM dogs, with the 2 poor-quality recordings being
due to inadequate alignment in highly spherical left ventricles.
For TMF, peak E was significantly higher in the OvDCM and OccDCM groups than in the NL group
(see Table 3 for all group comparisons). Age and HR were significant covariates such that E decreased
with increasing age and increased with increasing HR. All 3 groups differed significantly with respect to peak A, with OccDCM having the highest and OvDCM
having the lowest peak A. HR was the only significant covariate for peak A, with an increase in HR resulting in an increase in A velocity. The E : A ratio was significantly higher in
OvDCM compared with the other 2 groups. Age and BW were significant covariates, such
that E : A decreased with increasing age and E : A increased with increasing BW. DTE was significantly
different among all 3 groups, with the shortest DTE
in OvDCM and the longest DTE in NL. Significant covariates for DTE included age and BW, such that DTE increased with
increasing age and BW. IVRT had a complex model with sex, age, BW, and HR being significant covariates along with
some interactions (Table 3). IVRT was significantly longer in OccDCM compared with both NL and
OvDCM. NL and OvDCM did not differ.


IVRT increased with increasing age and BW and decreased with increasing HR. Females tended to
have
longer IVRTs than males, but this result depended on age and group.
Because of the number of missing data points for the PVF parameters, fewer covariates could be examined (ie,
fewer degrees of freedom). Specifically, in the case of peak S and S : D ratio, the models were
noted to be
overfitting the data (ie, all covariates significant and unreasonable LS means), therefore
the group-effect
alone (ANOVA) was examined for these 2 variables. Significant differences among groups were detected only
for antegrade flow. Peak S was significantly lower in OvDCM compared with both NL and OccDCM. Peak D was significantly
greater in OvDCM compared with both NL and OccDCM. S : D ratio was significantly different among all 3 groups, with OvDCM having
the lowest S : D ratio, and NL having the highest S : D ratio. No difference was detected among groups for peak AR, AR dur, or ratio of TMF A
duration to PVF AR duration.
Vp was significantly lower in OccDCM compared with NL. Vp in OvDCM was lower than normal, but not signifi-cantly different than the other 2 groups. There were no significant covariates in the model for Vp.
For TDI mitral annular velocities, Em was significantly lower in OvDCM compared with NL for themales. Am
was significantly different among all 3 groups, with lowest values in OvDCM and highest in NL,
resulting in E
m:Am ratio being significantly greater in OvDCM compared with NL and OccDCM. The systolic myocardial velocity, Sm, was
significantly different among all 3 groups (highest in NL, lowest in OvDCM). In OccDCM, 7/10 dogs reached a cardiac endpoint: 6 CHF and 1 SD. For the remaining 3 dogs, 2 experienced
noncardiac death (1 died secondary to hemorrhage from a pulmonary mass; 1 was euthanized for an intrathoracic mass), and 1 remained occult at the time of analysis. For those that
experienced CHF or SD, the mean time to endpoint was 177+132 days (mean + standard deviation),
with a median of 143 days and a range of 47–357 days. Of all the variables examined, only DTE
was significantly associated with time to onset of CHF or SD in OccDCM (RR 0.83, CI 0.7–0.98, P5 .03).

In OvDCM, 9/10 dogs experienced cardiac death: 5 were euthanized due to refractory CHF, and 4 experienced SD. The
remaining 1 dog was euthanized for appendicular osteosarcoma with stable CHF at the time. The mean time to cardiac death was 77+64 days, with a median of
62 days and a range of 13–214 days. Variables significantly associated with survival time included age
(RR 0.55, CI 0.29–0.94, P 5 .03), LVIDd (RR 1.15, CI 1.02–1.35, P5.02), and LVIDs (RR 1.24, CI 1.06–1.54, P5 .005). None of the
diastolic indices
were associated with survival time on bivariate analysis.

Discussion
Diastolic dysfunction in DCM may be the result of: (1) impaired relaxation due to abnormal calcium handling and abnormal kinetics of actin-myosin interactions, (2) increased myocardial stiffness
(reduced compliance) due to myocardial fibrosis, and (3) increased chamber stiffness due to cardiac remodelling and eccentric hypertrophy, resulting in operation of the LV on a steeper portion of
the passive diastolic pressurevolume curve. 3,26,27 Abnormal diastolic function plays a dominant role in the onset of CHF, as increased LV end-diastolic volume and pressure eventually lead to
increased pulmonary venous pressure and pulmonary edema. Increases in LV filling pressure correlate closely with congestive signs and exercise tolerance in humans, independent of the severity of
systolic dysfunction. 5,28 Evaluation of diastolic function is now an important part of monitoring human DCM patients, specifically for assessing prognosis and therapeutic response. There are few
reports describing assessment of diastolic function in clinical canine DCM patients,4,21,22 and none Table 3. Doppler echocardiography results by Analysis of Covariance: Least squares means with
95% confidence intervals. Normal Occult DCM Overt DCM Significant Covariates Transmitral flow n 5 10 n 5 10 n 5 10 E (cm/s) 71.6 (62.7–81.7) 96.9 (84.8–110.7)a 94.3 (82.8–107.3)a A,b HRc A (cm/s)
56.5 (50.7–62.9) 67.1 (60.0–75.1)a 47.7 (42.4–53.8)a, d HRc E : A 1.4 (1.2–1.6) 1.4 (1.3–1.6) 2.0 (1.8–2.2)e, d A,b BW,c BW 3 Gr A dur (milliseconds) 75.5 (68.0–82.9) 85.5 (79.0–92.1) 90.4
(82.1–98.8) A,b HRb DTE (milliseconds) 142.1 (129.5–154.7) 97.5 (86.5–108.5)e 79.0 (68.3–89.7)e, f A,c BWc Isovolumic Relaxation Time n 5 10 n 5 10 n 5 10 IVRT (milliseconds) M 76.4 (56.7–103.0)
95.4 (76.4–119.2)a 73.0 (61.5–86.7)f sex, A,c BW,c HR,b sex 3 A, A 3 BW, HR 3 Gr F 86.7 (74.2–101.4) 111.3 (90.6–136.9)a 73.0 (50.0–106.6)f Pulmonary venous flow n 5 10 n 5 9 n 5 5 S (cm/s) 50.6
(45.5–55.6) 44.2 (38.9–49.5) 34.4 (27.3–41.6)a, f n/a D (cm/s) 39.8 (31.0–48.5) 43.2 (33.3–53.1) 59.6 (48.7–70.5)a, f A,b BW,b HR,b A 3 Gr, BW 3 BW, BW 3 Gr S : D 1.0 (0.9–1.1) 0.8 (0.7–0.9)e 0.6
(0.5–0.7)e, f n/a AR (cm/s) 27.7 (25.0–30.7) 26.4 (23.6–29.5) 24.3 (20.9–28.4) HRc AR dur (milliseconds) 57.7 (52.9–62.5) 59.9 (54.8–65.0) 61.8 (55.0–68.6) A dur : AR dur 1.4 (1.3–1.6) 1.5
(1.3–1.7) 1.4 (1.2–1.6) Flow propagation velocity n 5 10 n 5 10 n 5 9 Vp (cm/s) 58.9 (48.9–70.9) 40.2 (33.4–48.4)a 45.6 (37.5–55.4) Lateral mitral annular velocities by TDI n 5 10 n 5 10 n 5 8 E
m (cm/s) M 19.5 (15.3–23.6) 15.3 (12.8–17.7) 14.9 (13.2–16.7)a A,b BW,c A 3 BW, sex 3 Gr F 14.9 (12.9–16.9) 17.0 (14.9–19.1) Not estimable Am (cm/s) M 9.2 (7.3–11.6) 7.1 (5.8–8.7)a 5.3
(4.6–6.0)e, f sex, BWc F 12.6 (10.8–14.8) 9.1 (7.7–10.7)a Not estimable Em : Am 1.5 (1.2–1.7) 1.9 (1.6–2.2) 2.6 (2.2–3.2)e, fSm (cm/s) 19.5 (17.3–21.9) 10.6 (9.4–11.9)e 7.5 (6.6–8.5)e, d BWc
Abbreviations are explained in the first footnote to Table 2; M, male; F, female; A, age; BW, body weight; Gr, group; n/a, not applicable (analysis of variance performed in place of analysis of
covariance due to too few observations). Data are presented as least squares mean (95% confidence interval). a P , .05 versus normal group. b Negative (inverse) relationship between variable and
covariate. c Positive (direct) relationship between variable and covariate. d P , .001 versus occult DCM group. e P , .001 versus normal group. fP , .05 versus occult DCM group. 86 O’Sullivan,
O’Grady, and Minors reporting the use of a combination of echocardiographic techniques designed to potentially overcome the limitations of individual indices. The results of this study provide
some insight into characterization of diastolic function in normal Doberman Pinschers and the
presence of diastolic dysfunction in Dobermans with DCM.
Diastolic function may be assessed noninvasively by echocardiographic examination of TMF because it reflects the instantaneous pressure differences between the left atrium (LA) and ventricle,
which in turn are related to the rate of myocardial relaxation and compliance of the 2 chambers.29 Abnormal patterns of TMF, although not specific for a particular disease, distinguish various
stages of diastolic dysfunction and change along a continuum with progression of myocardial disease. Early diastolic dysfunction is characterized by impaired LV relaxation, causing reduced early
filling (decreased E wave) and increased dependence on atrial contraction (increased A wave), resulting in E : A ratio
,1 and prolonged IVRT and DTE. With disease progression, LV compliance begins to decrease, and LA and LV filling pressures increase as a result, causing pseudonormalization of TMF (normal E : A
ratio, normal to short IVRT and DTE). In advanced diastolic dysfunction, there is very poor LV compliance, a further increase in LV filling pressure, and often atrial systolic dysfunction,
resulting in a restrictive TMF pattern (increased E wave, decreased A wave, E : A ratio $2,
and short IVRT and DTE).5,29,30
The normal Doberman Pinschers in this study had a similar E : A ratio but longer DTE compared with normal dogs of various breeds described in 2 other reports. 18,19 Differences in breed, BW, HR,
age, and FS among studies may account for these findings. The symptomatic Dobermans in this study (OvDCM) displayed features of a restrictive TMF pattern (E : A ratio $2, shortened DTE),
suggesting markedly reduced compliance and high filling pressures, consistent with advanced diastolic dysfunction. This conclusion is in agreement with findings in moderately to severely sympto-
matic human DCM patients, in whom higher E and lower A velocities, higher E : A ratios, and shorter DTE than asymptomatic or mildly symptomatic patients are reported. 31,32 As a group, the dogs
with occult DCM displayed features of multiple patterns such that the group did not fit neatly into one TMF pattern. This observation is likely the result of examining patients at different
stages of the occult disease process, in which varying degrees of abnormal relaxation and changes in compliance are occurring. 33 When examined individually, 1 dog had an impaired relaxa- tion
pattern (E : A ratio,1 and longest IVRT) and 1 dog tended toward a restrictive pattern (highest E : A ratio, short DTE,
and shortest IVRT), whereas the remaining dogs had some of the features of a pseudonormal pattern. Groups
of asymptomatic to mildly symptomatic humans typically demonstrate impaired relaxation or pseudonormal filling patterns, suggesting mild and moderate diastolic dysfunction, respectively. 31,32
The occult DCM dogs in this study represent a typical example of patients in which additional indices of diastolic function may be
necessary to draw conclusions.
Evaluation of PVF is a complementary means of assessing LV diastolic function. PVF is dependent on the pressure gradient between the PV and LA and consists of antegrade systolic flow (S)
associated with atrial relaxation and displacement of the mitral annulus apically during ventricular contraction, antegrade early diastolic flow (D) during LV filling, and retrograde late
diastolic flow (AR) associated with atrial contraction (Fig 2). 34,35 Most normal adult humans exhibit greater systolic than diastolic PVF (S : D ratio $1) and an AR wave ,35 cm/s,15,35 whereas
several reports in
normal dogs describe a tendency for greater diastolic than systolic antegrade PVF, resulting in an average S : D ratio ,1, but similar AR velocities. 18,19,36 In the present study, mean S : D
ratio in the normal group was 1, with 50% of the dogs having S : D ,1 and 50% .1. AR velocities were comparable with other studies. Potential reasons for higher S and S : D ratio in the normal
dogs of this study include differences in HR or sample volume depth within the PV compared with other studies.
Diastolic dysfunction produces alterations to PVF patterns. With impaired relaxation, LV pressure and, thus, LA pressure fall more slowly, resulting in reduced diastolic PVF, normal or increased
systolic flow, and S : D ratio .1. With disease progression, systolic PVF is blunted because of increased LA pressure and decreased LA compliance, diastolic PVF increases and decelerates rapidly
due to increased LA pressure and decreased LV compliance (resulting in an S : D ratio ,1), and velocity and duration of the AR wave increase. This pattern is observed with pseudonormal or
restrictive TMF pat- terns. However, if atrial systolic dysfunction is present with advanced disease, there may be loss of a prominent 15,29 AR wave in the face of restrictive filling. Blunted
systolic PVF is detected in 42–64% of human DCM 14,37,38 patients, and increased duration of PVF AR wave (specifically AR duration . TMF A duration) is reportedly a sensitive and specific marker
of increased 38,39 LV end-diastolic pressure. The dogs with DCM in this study exhibited reduced S : D ratios compared with the normal group, with the OvDCM group also exhibiting blunted systolic
PVF. This observation would appear to complement the TMF findings and provide further evidence of diastolic dysfunction. However, no significant differences were detected for retrograde PVF,
including the velocity and duration of the AR wave and ratio of TMF A duration to PVF AR duration. This lack of difference may be technical in origin given that the much smaller AR wave is more
difficult to record 24 and most likely to be disturbed by wall motion artifact. Atrial systolic dysfunction also may account for the lack of large AR waves in some dogs, particularly those in the
group with overt DCM. Recording of PVF proved to be technically challenging in dogs with DCM in this study, and adequate recordings were possible in 9/10 OccDCM and in only half of OvDCM.
Feasibility of PVF recording by experienced human sonographers is reported to range from 80–95% for antegrade flow, and
anywhere from only 40% up to 90% for retrograde 40,41 flow. Beginners may expect success in only 40–50% 6 of patients overall. Other factors that may have contributed to unobtainable recordings include cardiac enlargement, and hence depth limitation and distortion of the angle of PVF, poor image quality in some animals, wall motion artifact, presence of mitral re- gurgitation disrupting the S wave, and influence of respiration on cardiac image motion.
Vp is a preload-independent index of LV relaxation that has been validated in both humans and a canine 42 model. It is strongly negatively correlated with the time constant of LV relaxation, one potential gold standard 42–44 for assessment of LV relaxation. The normal value 15 for an adult human generally is .45 cm/s. Values in normal dogs were reported to range from 45 to 70 cm/s by 1 group, which is consistent with the findings in the 45 normal Dobermans in this study. A delay in LV flow propagation has been described in human patients with DCM and other cardiac diseases characterized by 43,46 diastolic dysfunction, and in an experimental canine 47 model of acute myocardial ischemia. This delay has 43,44,46,47 been linked directly to asynchrony of relaxation. In this study, Vp was significantly lower in OccDCM compared with NL, supporting the presence of impaired relaxation in occult DCM (Fig 3). Vp for OvDCM, while lower than NL and approaching that of OccDCM, was not statistically different from either group. Vp is proposed to be preload independent with LV relaxation being its primary determinant; however, other physio- logic determinants of Vp may include HR, age, and 42,46,48 percentage of segmental wall dyssynergy. The lack of significantly decreased Vp in OvDCM may be related to the small number of subjects studied and high variability in the measurement of this parameter.
The examination of myocardial motion with TDI is a novel means of assessing diastolic function. In the same way that Doppler echocardiography is used to record low amplitude, high velocity blood flow, it also may be applied to high amplitude, low velocity wall motion. Global LV diastolic function may be assessed from the mitral annular position in the apical 4-chamber view, and specifically longitudinal fiber shortening and 15 expansion are evaluated. The PW TDI display of mitral annulus motion appears as a mirror image of the TMF display, with early (Em) and late (Am) diastolic waves. A systolic wave (Sm) coincident with ventricular contraction also is present (Fig 4). A wide range of normal values has been reported for TDI indices in 49 humans. There is 1 report in the veterinary literature 20 on normal TDI values in dogs. TDI values obtained from the basal LV free wall in that report were lower than those for the normal Dobermans in this study, which may be related to the different methodology used. 20 Chetboul et al used color TDI and off-line analysis, generating average tissue velocity curves from the basal LV free wall myocardium, whereas this study used real- time PW TDI and measured peak velocities from the mitral annulus, which may yield higher values. The values in the normal dogs in this study are very similar 49 to those reported in humans obtained by PW TDI. An
interesting finding in the work by Chetboul et al a strong association between breed and TDI indices, with rather different values noted for different breed groups (Dobermans not reported), suggesting 20 breed-specific normal values may be warranted.
Human patients with various cardiac diseases having impaired relaxation as a component, including DCM, may have reduced Em velocities, independent of TMF 15,16 pattern. Earlier investigations suggested that Em was 16,50,51 independent of preload, whereas studies since that 52,53 time have challenged this assumption. It appears however that preload-dependence is much less pro- nounced in the presence of abnormal relaxation. This observation suggests that Em still is useful in differen- tiating normal from pseudonormal LV filling. In this study, Em was significantly reduced in the males in the overt phase of disease compared with the normal males. The clinical relevance of this finding is uncertain given the relatively small magnitude of difference and the large amount of overlap in values among groups. The other diastolic indices, including TMF, PVF, and Vp, suggest the presence of impaired relaxation in the DCM groups, hence it is problematic that TDI did not consistently indicate the same. TDI perhaps was insensitive in detecting abnormal relaxation for technical reasons because myocardial motion is caused not only by contraction and relaxation, but also by translation and rotation of cardiac structures, thus Em reflects more 15 than simply motion due to relaxation. Em velocities simply may be too variable, particularly in a small sample of dogs, to yield meaningful group differences. Translation-independent TDI indices such as the nega- tive myocardial velocity gradient, defined as the difference in early diastolic myocardial velocity between the endocardium and epicardium divided by myocardial wall thickness, are likely more sensitive indicators of 54 relaxation abnormalities. Accurate TDI indices may be difficult to obtain because of poor imaging and poor alignment with the LV free wall in dogs with dilated hearts, as was the case in 2 dogs in this study. Reduced Am velocities are found in patients with pseudonormal and restrictive TMF patterns as a result of increased LV end-diastolic pressure, which increases atrial afterload 17,53 and depresses atrial function. Our findings suggest progressively increasing atrial afterload and decreasing atrial function among the 3 groups (NL Am . OccDCM Am . OvDCM Am). Clearly, more efforts are required to establish normal diastolic TDI values in Dobermans and to elucidate the utility of these measures in this disease. Although not a primary objective of this study, systolic mitral annular velocities discriminated among the 3 groups, with reduced Sm in OccDCM, and further reduced Sm in OvDCM. Reduced Sm has been proposed in human patients to be a very sensitive and early 49,55,56 indicator of systolic dysfunction. At least 1 veterinary group has demonstrated the clinical utility of TDI in dogs. Chetboul et al found reduced early diastolic and systolic myocardial velocity gradients in preclinical Golden Retrievers with the dystrophin gene mutation for GRMD compared with normal Golden 4 Retrievers.
With respect to the prognostic utility of diastolic indices, DTE was significantly associated with time to onset of CHF or SD in OccDCM. For each unit decrease in DTE, there was a 17% increase in risk of CHF or SD. Shortening of DTE suggests rapid equilibration of LV and LA pressures during early filling because of decreased LV compliance. A short DTE (#125 milliseconds) has been described as the most powerful independent predictor of hospitalization for CHF and all-cause mortality in asymptomatic human patients with LV systolic dysfunction, regardless of E : A 12 ratio and systolic function indices. In an earlier study by the same group, DTE and pulmonary capillary wedge pressure (PCWP) were strongly negatively correlated in both asymptomatic and symptomatic LV systolic 57 dysfunction patients, again regardless of E : A ratio. Thus, a short DTE, suggesting decreased LV compliance and increased PCWP, should be predictive of the onset of CHF. Further investigation into the utility of this measurement and, in particular, serial measurements is warranted in a larger series of dogs. In the OvDCM group, age, LVIDd, and LVIDs were significantly associated with survival time. Younger age was associ- ated with poorer survival time, which has been reported 58 in at least 1 other study of DCM in dogs. Although it might be inferred that the disease may be more aggressive and rapidly progressive in younger animals, this has yet to be documented. Our study suggests a 15% and 24% increase in risk of death for every millimeter increase in LVIDd and LVIDs, respectively. There is little information in the veterinary literature available to corroborate the prognostic utility of echocardiography. A few retrospective studies have failed to find associa- tions between echocardiographic indices and survival 58,59 times, whereas Borgarelli et al suggested that in- creased LV end-systolic volume index (ESV-I) is 23 a significant predictor of poor survival. None of the diastolic parameters were individually predictive of survival in this study. This finding is in contrast to a multitude of information in the human literature supporting the utility of restrictive TMF patterns, short DTE, and blunted systolic PVF as predictors of poor prognosis. The lack of significant findings with respect to diastolic indices is likely a reflection of the small number of dogs in this study and the restriction to a rather simplistic attempt at elucidating associations through bivariate analysis.
Several limitations of this study require acknowledge- ment. A small number of dogs were included, and it is well recognized that small sample sizes are, by nature, underpowered to detect anything but substantive differences among groups. With respect to survival analysis in particular, we were limited to investigating the effect of single parameters alone. This study must be regarded, therefore, as a preliminary evaluation of the potential prognostic utility of these parameters. Follow- up studies in larger numbers of dogs are required. The results of this study are applicable to DCM in Dober- man Pinschers and may not represent the events occurring in other breeds. Echocardiographic indices of diastolic function are influenced by a number of factors that may contribute to variability. Technical factors including sonographer experience, sample vol- ume position, Doppler beam alignment, and gain and filter settings may affect diastolic measurements. Al- though every effort was made to standardize these factors, they may still have influenced the variability of the recordings. Physiologic factors including age, BW, HR, and sex were addressed in the analysis of co- variance such that group effects should not be con- founded by these known influences. All diastolic parameters were measured as close as possible to end- expiration with the use of a respiratory monitor, thus effects of respiration should have been minimized. Many of the diastolic indices, particularly TMF and PVF, are affected by loading conditions, thus varying degrees of mitral regurgitation and differences in medical therapy certainly may have influenced the results. Di- astolic function parameters are known to change over time during the course of disease; however, repeat echocardiographic evaluations were not performed for this study. Direct proof of increasing degrees of diastolic dysfunction across groups would involve follow-up and sequential measurement of the same dogs from the normal phase through the occult and overt phases of DCM. No invasive measurements were performed to provide de- finitive evidence of diastolic dysfunction; therefore con- clusions regarding the presence of diastolic dysfunction are presumptive, albeit based on data accepted as representative of diastolic function.
In conclusion, TMF, IVRT, and antegrade PVF were significantly different among the 3 groups of dogs and suggested increasing levels of diastolic dysfunction with disease progression. Shorter DTE was associated with shorter time to CHF or SD in the occult DCM group. Further investigation into the clinical utility of this parameter is warranted. PVF proved to be the most technically challenging of the diastolic indices, particu- larly the retrograde component and particularly in dogs with very enlarged hearts. As such, it is anticipated to have limited utility in the routine clinical assessment of canine DCM patients. Low Vp in the group with occult DCM distinguished this group from normal dogs and supported the presence of impaired relaxation. The utility of this index as an aid in the diagnosis of DCM may warrant further investigation. Diastolic mitral annular motion by TDI had a limited ability in distinguishing diseased dogs in this study. More extensive work in larger numbers of dogs is required to establish normal ranges and potential normal variability for these parameters.
Acknowledgments
Funding for this project was provided by The Pet Trust, Ontario Veterinary College, University of Guelph, Guelph, Ontario, Canada. The authors thank Rhonie Horne for technical assistance, William Sears and Gabrielle Monteith for statistical assistance, and all Doberman owners who participated in this study.
References
1. Wynne J, Braunwald E. The cardiomyopathies and myocar- ditides. In: Braunwald E, Zipes DP, Libby P, eds. Heart Disease: A Textbook of Cardiovascular Medicine. 6th ed. Philadelphia, PA: WB Saunders; 2001:1751–1806.
2. Sisson D, O’Grady MR, Calvert CA. Myocardial diseases of dogs. In: Fox PR, Sisson D, Moise NS, eds. Textbook of Canine and Feline Cardiology: Principles and Clinical Practice. 2nd ed. Philadelphia, PA: WB Saunders; 1999:581–619. 3. Grossman W. Diastolic dysfunction in congestive heart failure. N Engl J Med 1991;325:1557–1564.
4. Chetboul V, Carlos C, Blot S, et al. Tissue Doppler assessment of diastolic and systolic alterations of radial and longitudinal left ventricular motions in Golden Retrievers during the preclinical phase of cardiomyopathy associated with muscular dystrophy. Am J Vet Res 2004;65:1335–1341.
5. Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician’s Rosetta Stone. J Am Coll Cardiol 1997;30:8–18.
6. Rakowski H, Appleton C, Chan KL, et al. Canadian consensus recommendations for the measurement and reporting of diastolic dysfunction by echocardiography: From the Investiga- tors of Consensus on Diastolic Dysfunction by Echocardiography. J Am Soc Echocardiogr 1996;9:736–760.
7. Appleton CP, Firstenberg MS, Garcia MJ, Thomas JD. The echo-Doppler evaluation of left ventricular diastolic function: A current perspective. Cardiol Clin 2000;18:513–546, ix.
8. Nishimura RA, Abel MD, Hatle LK, et al. Significance of Doppler indices of diastolic filling of the left ventricle: Comparison with invasive hemodynamics in a canine model. Am Heart J 1989;118:1248–1258.
9. Spirito P, Maron BJ, Bonow RO. Noninvasive assessment of left ventricular diastolic function: Comparative analysis of Doppler echocardiographic and radionuclide angiographic techniques. J Am Coll Cardiol 1986;7:518–526.
10. Xie GY, Berk MR, Smith MD, et al. Prognostic value of Doppler transmitral flow patterns in patients with congestive heart failure. J Am Coll Cardiol 1994;24:132–139.
11. Pinamonti B, Di Lenarda A, Sinagra G, Camerini F. Restrictive left ventricular filling pattern in dilated cardiomyopathy assessed by Doppler echocardiography: Clinical, echocardiograph- ic and hemodynamic correlations and prognostic implications. Heart Muscle Disease Study Group. J Am Coll Cardiol 1993;22: 808–815.
12. Giannuzzi P, Temporelli PL, Bosimini E, et al. Independent and incremental prognostic value of Doppler-derived mitral deceleration time of early filling in both symptomatic and asymptomatic patients with left ventricular dysfunction. J Am Coll Cardiol 1996;28:383–390.
13. Temporelli PL, Corra U, Imparato A, et al. Reversible restrictive left ventricular diastolic filling with optimized oral therapy predicts a more favorable prognosis in patients with chronic heart failure. J Am Coll Cardiol 1998;31:1591–1597.
14. Dini FL, Dell’Anna R, Micheli A, et al. Impact of blunted pulmonary venous flow on the outcome of patients with left ventricular systolic dysfunction secondary to either ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 2000;85:1455– 1460.
15. Garcia MJ, Thomas JD, Klein AL. New Doppler echocar- diographic applications for the study of diastolic function. J Am Coll Cardiol 1998;32:865–875.
16. Farias CA, Rodriguez L, Garcia MJ, et al. Assessment of diastolic function by tissue Doppler echocardiography: Compari- son with standard transmitral and pulmonary venous flow. J Am Soc Echocardiogr 1999;12:609–617.
17. Nagueh SF, Middleton KJ, Kopelen HA, et al. Doppler tissue imaging: A noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol 1997;30:1527–1533.
18. Schober KE, Luis Fuentes V, McEwan JD, French AT. Pulmonary venous flow characteristics as assessed by transthoracic pulsed Doppler echocardiography in normal dogs. Vet Radiol Ultrasound 1998;39:33–41.
19. Schober KE, Fuentes VL. Effects of age, body weight, and heart rate on transmitral and pulmonary venous flow in clinically normal dogs. Am J Vet Res 2001;62:1447–1454.
20. Chetboul V, Sampedrano CC, Concordet D, et al. Use of quantitative two-dimensional color tissue Doppler imaging for assessment of left ventricular radial and longitudinal myocardial velocities in dogs. Am J Vet Res 2005;66:953–961.
21. Schober K, Fuentes VL. [Doppler echocardiographic assessment of left ventricular diastolic function in dogs] German. Tierarztl Prax Ausg K Klientiere Heimtiere 1998;26:13–20.
22. Minors SL, O’Grady MR. Resting and dobutamine stress echocardiographic factors associated with the development of occult dilated cardiomyopathy in healthy Doberman Pinscher dogs. J Vet Intern Med 1998;12:369–380.
23. Borgarelli M, Tarducci A, Santilli RA, et al. Echo prognostic indicators for DCM. Proceedings of the 18th Annual American College of Veterinary Internal Medicine Forum, Seattle, WA, May, 2000.
24. Appleton CP, Jensen JL, Hatle LK, Oh JK. Doppler evaluation of left and right ventricular diastolic function: A technical guide for obtaining optimal flow velocity recordings. J Am Soc Echocardiogr 1997;10:271–292.
25. Steel RGD, Torrie JH, Dickey DA. Analysis of covariance. In: Steel RGD, Torrie JH, Dickey DA, eds. Principles and Procedures of Statistics: A Biometrical Approach. 3rd ed. Boston, MA: McGraw-Hill; 1997:429–458.
26. Hunter WC. Role of myofilaments and calcium handling in left ventricular relaxation. Cardiol Clin 2000;18:443–457.
27. Solomon SB, Nikolic SD, Glantz SA, Yellin EL. Left ventricular diastolic function of remodeled myocardium in dogs with pacing-induced heart failure. Am J Physiol 1998;274:H945– 954.
28. Packer M. Abnormalities of diastolic function as a potential cause of exercise intolerance in chronic heart failure. Circulation 1990;81:III78–86.
29. Appleton CP, Hatle LK. The natural history of left ventricular filling abnormalities: Assessment by two-dimensional and Doppler echocardiography. Echocardiography 1992;9:437– 457.
30. Smith MD. Left ventricular diastolic function. In: Otto CM, ed. The Practice of Clinical Echocardiography. 2nd ed. Philadel- phia, PA: WB Saunders; 2002:113–140.
31. Vanoverschelde JL, Raphael DA, Robert AR, Cosyns JR. Left ventricular filling in dilated cardiomyopathy: Relation to functional class and hemodynamics. J Am Coll Cardiol 1990;15: 1288–1295.
32. Rihal CS, Nishimura RA, Hatle LK, et al. Systolic and diastolic dysfunction in patients with clinical diagnosis of dilated cardiomyopathy: Relation to symptoms and prognosis. Circulation 1994;90:2772–2779.
33. Oh JK, Appleton CP, Hatle LK, et al. The noninvasive assessment of left ventricular diastolic function with two-dimen- sional and Doppler echocardiography. J Am Soc Echocardiogr 1997;10:246–270.
34. Smallhorn JF, Freedom RM, Olley PM. Pulsed Doppler echocardiographic assessment of extraparenchymal pulmonary vein flow. J Am Coll Cardiol 1987;9:573–579.
35. Klein AL, Tajik AJ. Doppler assessment of pulmonary venous flow in healthy subjects and in patients with heart disease. J Am Soc Echocardiogr 1991;4:379–392.
36. Appleton CP. Hemodynamic determinants of Doppler pulmonary venous flow velocity components: New insights from studies in lightly sedated normal dogs. J Am Coll Cardiol 1997;30: 1562–1574.
37. Keren G, Sonnenblick EH, LeJemtel TH. Mitral annulus motion: Relation to pulmonary venous and transmitral flows in normal subjects and in patients with dilated cardiomyopathy. Circulation 1988;78:621–629.
38. Rossvoll O, Hatle LK. Pulmonary venous flow velocities recorded by transthoracic Doppler ultrasound: Relation to left ventricular diastolic pressures. J Am Coll Cardiol 1993;21:1687– 1696.
39. Yamamoto K, Nishimura RA, Burnett JC Jr, Redfield MM. Assessment of left ventricular end-diastolic pressure by Doppler echocardiography: Contribution of duration of pulmo- nary venous versus mitral flow velocity curves at atrial contraction. J Am Soc Echocardiogr 1997;10:52–59.
40. Brunazzi MC, Chirillo F, Pasqualini M, et al. Estimation of left ventricular diastolic pressures from precordial pulsed-Doppler analysis of pulmonary venous and mitral flow. Am Heart J 1994;128:293–300.
41. Jensen JL, Williams FE, Beilby BJ, et al. Feasibility of obtaining pulmonary venous flow velocity in cardiac patients using transthoracic pulsed wave Doppler technique. J Am Soc Echo- cardiogr 1997;10:60–66.
42. Garcia MJ, Smedira NG, Greenberg NL, et al. Color M- mode Doppler flow propagation velocity is a preload insensitive index of left ventricular relaxation: Animal and human validation. J Am Coll Cardiol 2000;35:201–208.
43. Brun P, Tribouilloy C, Duval AM, et al. Left ventricular flow propagation during early filling is related to wall relaxation: A color M-mode Doppler analysis. J Am Coll Cardiol 1992;20: 420–432.
44. Takatsuji H, Mikami T, Urasawa K, et al. A new approach for evaluation of left ventricular diastolic function: Spatial and temporal analysis of left ventricular filling flow propagation by color M-mode Doppler echocardiography. J Am Coll Cardiol 1996;27:365–371.
45. Schober KE. Diastolic function: Update & recent echocar- diographic advances. Proceedings of the 23rd Annual American College of Veterinary Internal Medicine Forum, Baltimore, MD, June, 2005.
46. Barbier P, Grimaldi A, Alimento M, et al. Echocardio- graphic determinants of mitral early flow propagation velocity. Am J Cardiol 2002;90:613–619.
47. Stugaard M, Smiseth OA, Risoe C, Ihlen H. Intraventric- ular early diastolic filling during acute myocardial ischemia, assessment by multigated color m-mode Doppler echocardiogra- phy. Circulation 1993;88:2705–2713.
48. Mego DM, DeGeare VS, Nottestad SY, et al. Variation of flow propagation velocity with age. J Am Soc Echocardiogr 1998;11:20–25.
49. Isaaz K. Tissue Doppler imaging for the assessment of left ventricular systolic and diastolic functions. Curr Opin Cardiol 2002;17:431–442.
50. Oki T, Tabata T, Yamada H, et al. Clinical application of pulsed Doppler tissue imaging for assessing abnormal left ventricular relaxation. Am J Cardiol 1997;79:921–928.
51. Sohn DW, Chai IH, Lee DJ, et al. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol 1997;30:474–480.
52. Firstenberg MS, Greenberg NL, Main ML, et al. Determi- nants of diastolic myocardial tissue Doppler velocities: Influences of relaxation and preload. J Appl Physiol 2001;90:299–307.
53. Nagueh SF, Sun H, Kopelen HA, et al. Hemodynamic determinants of the mitral annulus diastolic velocities by tissue Doppler. J Am Coll Cardiol 2001;37:278–285.
54. Shimizu Y, Uematsu M, Shimizu H, et al. Peak negative myocardial velocity gradient in early diastole as a noninvasive indicator of left ventricular diastolic function: Comparison with transmitral flow velocity indices. J Am Coll Cardiol 1998;32:1418– 1425.
55. Mishiro Y, Oki T, Yamada H, et al. Evaluation of left ventricular contraction abnormalities in patients with dilated cardiomyopathy with the use of pulsed tissue Doppler imaging. J Am Soc Echocardiogr 1999;12:913–920.
56. Nikitin NP, Witte KK. Application of tissue Doppler imaging in cardiology. Cardiology 2004;101:170–184.
57. Giannuzzi P, Imparato A, Temporelli PL, et al. Doppler- derived mitral deceleration time of early filling as a strong predictor of pulmonary capillary wedge pressure in postinfarction patients with left ventricular systolic dysfunction. J Am Coll Cardiol 1994;23:1630–1637.
58. Tidholm A, Svensson H, Sylven C. Survival and prognostic factors in 189 dogs with dilated cardiomyopathy. J Am Anim Hosp Assoc 1997;33:364–368.
59. Monnet E, Orton EC, Salman M, Boon J. Idiopathic dilated cardiomyopathy in dogs: Survival and prognostic indicators. J Vet Intern Med 1995;9:12–17.
60. Faris R, Coats AJ, Henein MY. Echocardiography-derived variables predict outcome in patients with nonischemic dilated cardiomyopathy with or without a restrictive filling pattern. Am Heart J 2002;144:343–350.
61. Hansen A, Haass M, Zugck C, et al. Prognostic value of Doppler echocardiographic mitral inflow patterns: Implications for risk stratification in patients with chronic congestive heart failure. J Am Coll Cardiol 2001;37:1049–1055.
62. Werner GS, Schaefer C, Dirks R, et al. Prognostic value of Doppler echocardiographic assessment of left ventricular filling in idiopathic dilated cardiomyopathy. Am J Cardiol 1994;73:792–798.
Share this article / Teilen Sie diesen Artikel
Diese Webseite wurde mit Jimdo erstellt! Jetzt kostenlos registrieren auf https://de.jimdo.com








