R&D Systems, Minneapolis, MN

Article added / Artikel hinzugefügt 01.10.2021
Generally Articles and Discussions about Osteosarcoma in Dogs
→ Evaluations of phylogenetic proximity in a group of 67 dogs with
osteosarcoma: a pilot study
Article added / Artikel hinzugefügt 01.10.2021
Generally Articles and Discussions about Osteosarcoma in Dogs
→ Canine Periosteal Osteosarcoma
Images added / Abbildungen hinzugefügt 02.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Pulmonary vessels
New subcategory added / Neue Unterkategorie hinzugefügt 02.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Pulmonary vessels
Images added / Abbildungen hinzugefügt 01.05.2019
Generally Sonography Atlas of Dogs →
Cardiovascular system → Heart valvular diseases



Pro-tumorigenic Effects of Transforming Growth Factor Beta 1 in Canine Osteosarcoma

Portela, R.F., Fadl-Alla, B.A., Pondenis, H.C., Byrum, M.L., Garrett, L.D., Wycislo, K.L., Borst, L.B. and Fan, T.M. (2014), "Pro-tumorigenic Effects of Transforming Growth Factor Beta 1 in Canine Osteosarcoma" . Journal of
Veterinary Internal Medicine, 28: 894–904.
doi: 10.1111/jvim.12348

Abstract
Background
Transforming growth factor beta 1 (TGFβ1) is a pleiotropic cytokine that contributes to reparative skeletal remodeling by inducing osteoblast proliferation, migration, and angiogenesis. Organic bone matrix is the largest bodily reservoir for latent TGFβ1, and active osteoblasts express cognate receptors for TGFβ1 (TGFβRI and TGFβRII). During malignant osteolysis, TGFβ1 is liberated from eroded bone matrix and promotes local progression of osteotropic solid tumors by its mitogenic and prosurvival activities.
Hypothesis
Canine osteosarcoma (OS) cells will possess TGFβ1 signaling machinery. Blockade of TGFβ1 signaling will attenuate pro-tumorigenic activities in OS cells. Naturally occurring primary OS samples will express cognate TGFβ1 receptors; and in dogs with OS, focal malignant osteolysis will contribute to circulating TGFβ1 concentrations.
Animals
Thirty-three dogs with appendicular OS.
Methods
Expression of TGFβ1 and its cognate receptors, as well as the biologic effects of TGFβ1 blockade, was characterized in OS cells. Ten spontaneous OS samples were characterized for TGFβRI/II expressions by immunohistochemistry. In 33 dogs with OS, plasma TGFβ1 concentrations were quantified and correlated with bone resorption.
Results
Canine OS cells secrete TGFβ1, express cognate receptors, and TGFβ1 signaling blockade decreases proliferation, migration, and vascular endothelial growth factor secretion. Naturally occurring OS samples abundantly and uniformly express TGFβRI/II, and in OS-bearing dogs, circulating TGFβ1 concentrations correlate with urine N-telopeptide excretion.
Conclusions and Clinical Importance
Canine OS cells possess TGFβ1 signaling machinery, potentially allowing for the establishment of an autocrine and paracrine pro-tumorigenic signaling loop. As such, TGFβ1 inhibitors might impede localized OS progression in dogs.
- NTx N-telopeptide
- OS osteosarcoma
- TGFβ1 transforming growth factor beta 1
Transforming growth factor beta (TGFβ) is a pleiotropic cytokine expressed in a variety of tissues. Three distinct TGFβ isoforms (TGFβ1, β2, or β3) have been identified, and exert their activities through receptor serine-threonine kinases.[1, 2] Secreted as latent homodimer precursors, TGFβ isoforms require catalysis by proteases, such as plasmin and matrix metalloproteinases,[3, 4] to be fully activated.[1, 5] Of the 3 isoforms, TGFβ1 exists in highest concentrations in circulation, with platelets and bone matrix serving as the 2 largest biologic sources of TGFβ1 within the body.[6, 7]
Intracellular signaling mediated by TGFβ requires the heterodimerization of its 2 cognate receptor serine-threonine kinases, TGFβRI and TGFβRII.[1, 2, 8] Biologic effects exerted by TGFβ are mediated by 2 divergent signaling pathways, Smad-dependent (canonical) and Smad-independent (noncanonical). The canonical signaling pathway is well characterized and necessitates the phosphorylation and subsequent colocalization of Smad partnering complexes to the nucleus, which then serve as transcription factors for TGFβ-mediated target genes.[1, 2, 8-10] The noncanonical pathway is less well defined, but this signaling pathway contributes to cell proliferation, motility, and survival by activation of the mitogen-activated protein kinase, Rho GTPase, and PI3K/AKT pathways, respectively.[9, 10]
Physiologic activities of TGFβ include regulation of skeletal health and remodeling. Normally, osteoblasts actively secrete TGFβ1, which becomes incorporated into the hydroxyapatite matrix of bone, and is subsequently released into the local microenvironment and systemic circulation after osteoclast-mediated bone resorption.[11-13] During reparative or pathologic bone remodeling, TGFβ1 concentrations transiently increase as a result of excessive osteoclast activity and consequent release of bone-derived TGFβ1.[14, 15] Within regions of reparative skeletal healing, released TGFβ1 can promote osteoblast precursor recruitment and motility, osteoblast proliferation and differentiation, as well as osteoblast survival.[16-19] Upon completion of bone healing and attenuated osteoclastic activities, the release and pro-osteoblastic activities of TGFβ1 become self-limiting.
Canine osteosarcoma (OS) is the most common primary bone tumor in dogs.[20] Clinically, affected dogs initially present for severe bone pain as a consequence of aggressive focal malignant osteolysis.[21-23] If localized pain is adequately controlled with interventional therapies, the majority of affected dogs eventually will develop distant metastatic disease.[24-27] Osteosarcoma progression within bone or distant organs can be rationally attributed to the unchecked growth of malignant osteoblasts, and although conventional therapies, including limb amputation and systemic chemotherapy, improve overall survival times of affected dogs, there remains a clinical need for biologically driven discovery of novel therapeutic targets.
In the context of canine OS, inhibiting cellular pathways involved in osteoblast proliferation, migration, and survival might lead to therapeutic improvements. Given the pivotal role of TGFβ1 in reparative bone biology, in conjunction with TGFβ1's participation in diverse tumorigenic processes including growth, migration, and angiogenesis,[28-30] we sought to investigate the potential role of this signaling axis in canine OS. The specific aims of this study were (1) to characterize TGFβ-associated ligand/receptor expressions in OS cell lines, spontaneous tumor samples, and OS-bearing dogs; (2) to study the in vitro effects of TGFβ signaling blockade on malignant osteoblast proliferation, migration, survival, and vascular endothelial growth factor (VEGF) secretion; and (3) to evaluate the contribution of malignant osteolysis to circulating TGFβ1 concentrations in dogs diagnosed with OS.
Materials and Methods
Cell Lines
Five canine (POS, HMPOS, COS31, Abrams, and D17) OS cell lines, 1 human (143B) OS cell line, 1 normal canine osteoblast cell line (K9OB), and a human pulmonary carcinoma cell line (A549) were used in this study. The POS and HMPOS cell lines were provided by James Farese, University of Florida, and the COS31 line was provided by Ahmed Shoieb, University of Tennessee. The K9OB cell line was established from a fragment of normal canine trabecular bone at the University of Illinois, Comparative Oncology Research Laboratory. All other cell lines were purchased commercially from American Tissue Culture Collection (Manassas, VA). All cell lines were cultured in either RPMI1640 or DMEM, with 10% fetal bovine serum and 1% penicillin/streptomycin. Cell cultures were maintained at 37°C in 5% CO2 and passaged twice weekly.
Reagents and Antibodies
Human recombinant TGFβ11 was purchased and used for Smad phosphorylation experiments. A selective TGFβRI/II inhibitor, LY2109761,2 was purchased for in vitro inhibition studies. Antibodies purchased included mouse monoclonal antihuman Smad 2/3 antibody,3 rabbit monoclonal antihuman phosphorylated-Smad 2 antibody,4 rabbit polyclonal antihuman TGFβRI antibody,5 rabbit polyclonal antihuman TGFβRII antibody,5 rabbit polyclonal antimouse Phosphorylated-Akt antibody,6 rabbit polyclonal antimouse Akt antibody,6 rabbit polyclonal antihuman β-actin antibody,5 and mouse monoclonal antihuman HIF-1α antibody.5 Although the majority of antibodies utilized were validated for use in dogs based upon manufacturer specifications, specifically for TGFβRI and TGFβRII antibodies, canine cross-reactivity for western blot analysis and immunohistochemistry was supported by the detection of proteins of expected molecular weight and subcellular localization, respectively, in conjunction with the use of known human positive controls.
Western Blot Analysis
Protein Extraction and Loading Methodologies
For protein extraction, cell lines were grown to confluence in 100 mm petri dishes, and then scraped, harvested, and pelleted by centrifugation. Subsequently, proteins were extracted using a commercial reagent (M-PER Assay7), mixed with a protease inhibitor cocktail,8 and concentrations quantified using a commercial kit (BCA Assay7). For all western blots conducted, 50 μg of protein was loaded and separated by SDS-PAGE using 10% polyacrylamide gels and transferred onto nitrocellulose membranes, with blocking at room temperature in 5% nonfat dry milk TBS-Tween immediately before incubation with target primary antibodies in 5% nonfat dry milk TBS-Tween overnight at 4°C; and after primary antibody incubation, membranes were washed and incubated for 1 hour with appropriate horseradish peroxidase–conjugated secondary antibodies (1 : 1,000) in 5% nonfat dry milk TBS-Tween and developed using a standard chemiluminescence detection kit.9
TGFβRI/II
Protein from 8 cell lines (5 canine OS, 1 normal canine osteoblast, 1 human OS, and 1 human lung carcinoma) was extracted and loaded as described. Membranes were incubated with either antihuman TGFβRI or TGFβRII antibodies (1:1000) in 5% nonfat dry milk TBS-Tween overnight at 4°C.
Smad 2/3 and Phosphorylated-Smad 2
The HMPOS cell line was grown to 80% confluence, and then serum starved with or without inclusion of LY2109761 for 24 hours. Protein was collected from 5 experimental conditions, including (1) serum starved for 24 hours only or serum starved for 24 hours and then stimulated for 1 hour with (2) 10% FBS media only; (3) 10% FBS media + human recombinant TGFβ1 (5 ng/mL); (4) 10% FBS media + LY2109761 (5 μM); and (5) 10% FBS media + human recombinant TGFβ1 (5 ng/mL) + LY2109761 (5 μM). Membranes were incubated with either antihuman Smad 2/3 antibody (1 : 500) or antihuman phosphorylated-Smad 2 antibody (1 : 1,000) in 5% nonfat dry milk TBS-Tween overnight at 4°C.
Akt and Phosphorylated Akt
The HMPOS cell line was grown in complete media and then serum starved with or without inclusion of LY2109761 for 24 hours. Protein was collected from four experimental conditions, including (1) serum starved for 24 hours only or serum starved for 24 hours and then stimulated for 1 hour with (2) human recombinant TGFβ1 (5 ng/mL); (3) LY2109761 (5 μM) alone; (4) LY2109761 (5 μM) + human recombinant TGFβ1 (5 ng/mL). Membranes were incubated with either a rabbit polyclonal antimouse phosphorylated-Akt antibody or a rabbit polyclonal antimouse Akt antibody, both at a 1 : 1,000 dilution.
HIF-1α and β-actin
The Abrams cell line was grown in complete media to confluence and then exposed to LY2109761 (5 μM) for varying exposure durations. Membranes were incubated with a mouse monoclonal antihuman HIF-1α antibody (1 : 500). Subsequently, membranes were stripped and reprobed with a rabbit polyclonal antihuman β-actin antibody (1 : 5,000) and HRP-conjugated antirabbit secondary antibody (1 : 1,000). Quantitative dosimetry of HIF-1α relative to β-actin was performed using Image J software and expressed as a ratio.
Immunohistochemistry
Antibody Validation using Cell Pellets
Five canine OS cell lines and a human positive control cell line, A549,[31] were used to validate the application of TGFβRI/II antibodies for immunohistochemical staining of formalin-fixed paraffin-embedded samples. Adherent cell cultures were collected and washed in PBS, then pelleted by centrifugation. Each pellet was resuspended in 1 mL 10% formalin for 1 hour. Formalin was removed and cell pellets were resuspended uniformly into 1 mL 4% melted agarose gel by vortexing, and then immediately centrifuged to create an agarose-embedded cell pellet. Cell pellets then were trimmed and processed by an identical protocol routinely used for formalin-fixed paraffin-embedded tissue biopsy specimens. Briefly, paraffin-embedded cell pellets were sectioned every 3 μm and placed on positively charged slides, and dried for 1 hour at 60°C. Slides were deparaffinized with 3 sequential xylene washes, and subsequently hydrated using 100, 95, and 70% ethanol for 2 minutes each; followed by a water rinse. Slides were placed in 3% hydrogen peroxide in methanol for 15 minutes, and then treated with citrate buffer (pH 6.0) at a temperature of 95°C for 10 minutes. SuperSensitive Wash Buffer and Peroxide block was applied for 10 minutes at room temperature to block nonspecific staining. The rabbit polyclonal antihuman TGFβRI and TGFβRII antibodies were used at a concentration of 1 : 100 for 1 hour at room temperature. Slides were rinsed with SuperSensitive Wash Buffer and treated with Super Enhancer for 20 minutes at room temperature, and subsequently treated with Polymer-HRP for 30 minutes at room temperature and incubated with 3,3′-diaminobenzidine (DAB) at room temperature for 5 minutes. Slides were washed with SuperSensitive Wash Buffer and counterstained with Mayer's hematoxylin for 1 minute. Staining intensity for each canine OS cell line was qualitatively compared with the staining intensity of the human positive control using an arbitrary subjective integer scale: (−) no staining; (+) light uniform staining; (++) moderate uniform staining; and (+++) intense uniform staining.
Spontaneous OS Samples
Ten canine appendicular OS tissue blocks were provided by Dr. Luke Borst for immunohistochemical assessment. Slides were deparaffinized in xylene and rehydrated in ethanol. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol for 15 minutes, and then slides were rinsed twice with wash buffer for 5 minutes. Slides were incubated with preheated 0.1% protease at 37°C for 20 minutes, and then rinsed in wash buffer for 2 minutes. Nonspecific staining was minimized by blocking for 10 minutes with Power Block, and then blocking for 15 minutes with avidin and biotin block. Blocked slides were incubated with rabbit polyclonal antihuman TGFβRI and TGFβRII antibodies at a concentration of 1 : 100 for 1 hour at room temperature. Slides were incubated with a biotinylated secondary antibody for 20 minutes at room temperature; then washed in buffer before incubation for 20 minutes with a streptavidin-biotinylated horseradish peroxidase complex, and developed with DAB substrate for 5 minutes and counterstained with hematoxylin. The negative control for the samples was processed identically in the absence of the primary antibody.
Colony-Forming Assay
The Abrams and HMPOS cell lines were seeded overnight into 6-well plates at a concentration of 50 cells per 3 mL of DMEM with 10% FBS. Upon adherence of individual cells, media was discarded and new media containing different experimental conditions was added, including DMEM 5% FBS only or DMEM 5% FBS with vehicle (DMSO) or a range of LY2109761 concentrations (0.3–5 μM). Cells were allowed to grow undisturbed for 7 (Abrams) or 10 (HMPOS) days. Subsequently, wells containing colonies were gently rinsed with chilled PBS, treated with 3 mL of solution containing 6.0% glutaraldehyde and 0.5% crystal violet for 30 minutes, and then rinsed with tap water. The number of visible colonies and the average size of colonies per experimental condition were quantified using imaging software.10 Two independent experiments were performed, with duplicates conducted for each experimental condition.
Scratch Assay
The Abrams, D17, and HMPOS cell lines were grown to 80% confluence in 6-well plates in DMEM supplemented with 10% FBS (complete media). Six experimental conditions for a duration of 48 hours were evaluated, including complete media only or complete media with vehicle (DMSO) or a range of LY2109761 concentrations (0.3–5 μM). A standardized acellular gap was created through cell monolayers using a 200-μL pipette tip in the middle of each well. Images of the acellular gap were captured at time 0 (maximal gap) and 48 hours for each experimental condition and analyzed with imaging software.10 The average width of 5 representative acellular gaps per experimental condition and cell line was used for quantitative comparisons at 48 hours, and 3 independent experimental replicates were performed.
VEGF ELISA
The Abrams, D17, and HMPOS cell lines were plated in complete media at 37°C and 5% CO2 with a seeding density of 5,000 cells per well in a 96-well plate. After adherence, complete media was removed and replaced with 1% FBS DMEM with either vehicle (DMSO) or various concentrations of LY2109761 (0.3–5 μM) for 48 hours. Cell culture supernatants were collected and the concentration of secreted VEGF was quantified using a commercial kit (Canine VEGF Quantikine ELISA Kit1) and normalized for cell proliferation by use of a colorimetric proliferation assay.11 Three independent experiments were performed, with hexaplicates conducted for each experimental condition.
TGFβ1 ELISA
The Abrams, D17, and HMPOS cell lines were incubated with 1% FBS DMEM at different cell densities ranging from 25 to 100 × 103 cells per well for 24 hours. Cell culture supernatants were collected and total TGFβ1 was determined with a commercially available ELISA kit (Quantikine® Mouse/Rat/Porcine/Canine TGFβ1 Assay1). In 33 dogs with appendicular OS, heparinized plasma was collected pretreatment and 28 days after institution of standardized palliative radiation treatment and IV zoledronate. Plasma samples were stored at −80°C and batch analyzed using a commercially available ELISA kit (Quantikine® Mouse/Rat/Porcine/Canine TGFβ1 Assay1).
Urine N-Telopeptide (NTx) assay
Pretreatment morning urine was collected from 33 dogs treated with standardized radiation therapy and IV zoledronate. Urine samples were immediately centrifuged at 4°C at 250 × g for 10 minutes, and the supernatant was collected and stored at −20°C before analysis was performed. Urine creatinine and NTx concentrations were measured with commercial ELISA test kits (Parameter Creatinine ELISA1).12
Statistical Analysis
Data sets were assessed for normality using the Shapiro-Wilk test. For all data comparisons evaluating the effect of TGFβRI/II kinase blockade with LY2109761, a 1-ANOVA and posthoc Dunnet's test were used to identify significant differences among experimental conditions compared with DMSO vehicle control. In dogs with OS, Pearson correlation coefficient was used to characterize any relationship between pretreatment plasma TGFβ1 and urine N-telopeptide concentrations and a 2-sided, paired t-test was utilized to determine the significance of changes in plasma TGFβ1 concentrations before and after treatment with standardized radiation therapy and IV zoledronate. Significance was defined as P < .05.
Results
TGFβRI/II Expression and TGFβ1 Secretion by OS Cell Lines
By western blot analysis, OS cells derived from human and canine origin demonstrated the presence of both TGFβRI and TGFβRII (Fig 1A). Subjectively, TGFβRI was more robustly detectable in comparison with TGFβRII after normalizing for β-actin loading control. As expected based upon the participatory role of TGFβ in homeostatic skeletal biology, normal canine osteoblasts also expressed TGFβRI and TGFβRII. Expression of TGFβRI and TGFβRII also was identified in OS cell lines by immunohistochemistry (Fig 1B), demonstrating a membranous and cytosolic staining pattern consistent with the cellular localization of receptor serine-threonine kinases. Active secretion of the cognate ligand, TGFβ1, was demonstrated by titration studies in three canine OS cell lines (Fig 1C). At the highest cell densities evaluated (approximately 105 cells), total TGFβ1 concentrations achieved physiologically relevant and active concentrations in the ng/mL range. The HMPOS cell line demonstrated the greatest capacity to secrete TGFβ1, approximately doubling or quadrupling the concentrations liberated by Abrams and D17, respectively.
Figure 1. In a panel of immortalized cell lines, protein detection by (A) western blot and (B) immunohistochemistry identifies the expressions of TGFβRI and TGFβRII in OS cell lines. Tabulated summary represents qualitative positive staining relative to A549 positive control as detected by immunohistochemistry. By enzyme-linked immunosorbent assay (C), spontaneous secretion of TGFβ1 by canine OS cell lines is confirmed by titration studies that demonstrate a direct relationship between cell density and supernatant TGFβ1 concentrations. Data expressed as mean ± SD.

LY2109761 Blocks Canonical TGFβ Signaling in Canine OS Cells
To confirm the capacity of LY2109761 to block the canonical signaling pathway of TGFβ in malignant canine osteoblasts, HMPOS cells were exposed to 5 different in vitro conditions with subsequent characterization of phosphorylated-Smad 2 and Smad 2/3 by western blot analysis (Fig 2). Under serum starved conditions only, the HMPOS cell line demonstrated detectable, but low, concentrations of phosphorylated-Smad 2. Stimulation for 1 hour with 10% FBS increased phosphorylated-Smad 2 concentrations, whereas combined stimulation with 10% FBS and recombinant TGFβ1 markedly induced Smad 2 phosphorylation. The addition of 5 μM LY2109761 completely blocked 10% FBS-elicited Smad 2 phosphorylation, and substantially attenuated Smad 2 phosphorylation induced by concurrent stimulation with 10% FBS and recombinant TGFβ1.
Figure 2. Western blot analysis demonstrating functional Smad-dependent signaling mediated through TGFβRI and TGFβRII in HMPOS canine OS cells. Increases in phosphorylated Smad 2 are induced by stimulation with either 10% fetal bovine serum alone or in combination with exogenous human recombinant TGFβ1. Pretreatment with LY2109761 (5 μM) completely prevents or attenuates Smad 2 phosphorylation after stimulation and substantiates the capacity for LY2109761 to block TGFβ signaling in canine cells.

LY2109761 Attenuates Pro-tumorigenic Activities in Canine OS Cells
Given the participatory role of TGFβ signaling in multiple facets of cancer progression, the potential anticancer effects of LY2109761, a highly potent and selective small molecule inhibitor of TGFβRI (Ki 0.04 μM) and TGFβRII (Ki 0.3 μM), on canine OS cell proliferation, migration, survival, and angiogenesis were investigated. With the capacity to establish distinct, noncoalescencing cell colonies in vitro, HMPOS and Abrams cell lines were selected to investigate the effect of LY2109761 on cell proliferation as evaluated by the number and size of colonies formed. Starting with an initial seed density of 50 cells per well, the antiproliferative effects of LY2109761 (0.3–5 μM) were characterized in HMPOS and Abrams cell lines by 7-day and 10-day colony-forming assays, respectively. For HMPOS cells, 10-day incubation with complete media with vehicle control (DMSO) resulted in an average generation of 265 ± 4 colonies. The addition of LY2109761 significantly decreased the number of colonies formed for all concentrations tested in comparison with vehicle control (Fig 3A,B), P < .01 for all comparisons. The average numbers of colonies formed after incubation with LY2109761 at each respective concentration were 204 ± 2 (0.3 μM), 200 ± 3 (1 μM), 139 ± 2 (3 μM), and 65 ± 1 (5 μM). A similar, but less substantial, reduction in cell proliferation was elicited by LY2109761 in the Abrams cell line (Fig 3C). The average numbers of colonies formed for each experimental condition were 503 ± 7 (DMSO), 483 ± 5 (0.3 μM), 460 ± 42 (1 μM), 392 ± 4 (3 μM), and 344 ± 5 (5 μM). Paralleling the observed reduction in absolute number of colonies formed, higher concentrations of LY2109761 also modestly diminished the maximal diameter of cell colonies formed (Fig S1).
Figure 3. Visually evident (A) and quantifiable (B) dose-dependent antiproliferative effects exerted by LY2109761 in the HMPOS cell line demonstrated by colony-forming assay after 10 days of undisturbed growth. Similar quantitative (C) decreases in colony formation with LY2109761 exposure in the Abrams cell line after 7 days of undisturbed growth. Data expressed as mean ± SD. *P < .05 and **P < .01 in comparison with DMSO control.

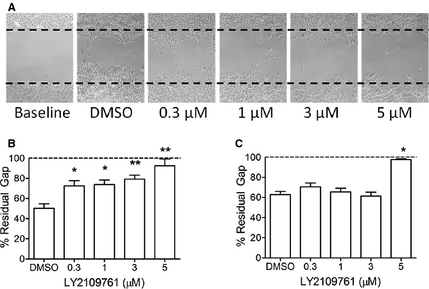
In addition to cell proliferation, the migration of malignant osteoblasts was attenuated by blocking TGFβ signaling. The extent of migratory inhibition, however, was highly cell lineage–dependent, represented in order of greatest to least inhibited being D17, HMPOS, and Abrams. The migration of the D17 cell line was most robustly inhibited by LY2109761 at all concentrations tested (Fig 4A,B), with percentage of maximal gap remaining after 48 hours of incubation being 50.2 ± 9.9% (DMSO), 72.6 ± 11.3% (0.3 μM), 73.8 ± 10.4% (1 μM), 79.1 ± 8.9% (3 μM), and 92.3 ± 14.9% (5 μM). Unlike the marked reductions in cell migration observed in D17 cells, only the highest concentration of LY2109761 was capable of slowing the rate and extent of migration relative to vehicle control in HMPOS cells, with percentage of maxi-mal gap remaining after 48 hours of incuba-tion being 62.8 ± 7.4% (DMSO), 70.6 ± 8.3% (0.3 μM), 65.6 ± 7.1% (1 μM), 61.3 ± 8.5% (3 μM), and 97.5 ± 1.5% (5 μM); (Fig 4C). The effect of LY2109761 on Abram cell migration was marginal, and even the 2 highest concentrations of 3 μM and 5 μM only maintained a percentage of maximal gap of 11.0 ± 1.9% and 20.3 ± 5.9%, respectively.
Figure 4. Visually evident (A) and quantifiable (B) dose-dependent inhibition of migration exerted by LY2109761 48 hours after scratch formation in D17 monolayer. Similar quantitative (C) attenuations in cell migration with LY2109761 exposure 48 hours after scratch formation in the HMPOS monolayer. Data expressed as mean ± SD. *P < .05 and **P < .01 in comparison with DMSO control.
The ability of LY2109761 to modulate the prosurvival Akt pathway was evaluated in the Abrams cell line by western blot assessment of phosphorylated Akt and Akt. Even under nonstimulatory and serum starved conditions, phosphorylated Akt was robustly expressed, and stimulation with recombinant TGFβ1 (5 ng/mL) or inhibition with LY2109761 (5 μM) did not alter the extent of phosphorylated Akt relative to total Akt (data not shown).
Given the pro-angiogenic properties of TGFβ signaling, the effect of LY2109761 on VEGF secretion by D17, HMPOS, and Abrams cell lines was studied. Consistently, LY2109761 exerted a dose-dependent reduction in normalized secreted VEGF, generally at concentrations exceeding 1 μM (Fig 5A). At the highest concentration of LY2109761, the secretion of VEGF was decreased by 40–45% in comparison with DMSO control for all cell lines tested. Canonical signaling through the TGFβ pathway increases hypoxia-inducible factor 1-alpha (HIF-1α) protein stability by downregulation of HIF-1α-associated prolyl hydroxylase (PHD), a key enzyme involved in targeting HIF-1α for proteasome degradation. To provide a plausible mechanism for the observed reductions in secreted VEGF after exposure to LY2109761, a relationship between TGFβ signaling inhibition and decreased HIF-1α protein stability was investigated. The Abrams OS cell line, which demonstrated the greatest reduction in secreted VEGF after LY2109761 exposure, was grown to confluence, serum starved overnight, and then incubated with 5 μM LY2109761 for different exposure durations (0–60 minutes). In comparison with untreated cells (time 0 minutes), HIF-1α concentrations decreased as a function of exposure time to LY2109761 with maximal reductions in HIF-1α approaching 35% after 60 minutes (Fig 5B,C). Relative reductions in HIF-1α protein were mediated by LY2109761 and were not simply a by-product of DMSO exposure, because vehicle control experiments did not result in any change in HIF-1α protein as a function of exposure time (data not shown).
Figure 5. Quantification (A) of LY2109761 dose-dependent decrease in normalized secreted VEGF in 3 canine OS cell lines as measured by enzyme-linked immunosorbent assay. In Abrams OS cells, LY2109761 (5 μM) induces visually evident (B) and quantifiable (C) decreases in HIF-1α protein after brief exposure durations. Data expressed as mean ± SD. *P < .05 and **P < .01 in comparison with DMSO control for VEGF secretion, or in comparison with time 0 minutes for HIF-1α expression.

TGFβ Axis in Dogs with OS
To expand upon the potential clinical relevance of the TGFβ signaling axis in dogs with OS, the expression of TGFβRI and TGFβRII was investigated in 10 primary canine osteoblastic OS samples. Similar to canine OS cell lines, malignant osteoblasts in all tumor samples consistently stained positive for TGFβRI and TGFβRII (Fig 6A). Given the focal malignant osteolytic nature of canine OS, in conjunction with bone matrix serving as the largest source of TGFβ in the mammalian body, a correlation between pretreatment plasma TGFβ1 concentration and urine N-telopeptide excretion was investigated in 33 dogs with appendi-cular OS. The average concentrations for urine N-telopeptide excretion and plasma TGFβ1 were 191.1 ± 69.4 nM BCE/mM creatinine and 18.6 ± 8.7 ng/mL, respectively. Consistent with the notion that TGFβ1 might be released into circulation as a consequence of focal malignant osteolysis, a positive correlation was identified between pretreatment plasma TGFβ1 and urine N-telopeptide excretion (Fig 6B); (r = 0.36; P = .04). To further substantiate that focal malignant osteolysis associated with OS might contribute to circulating TGFβ1 concentrations, serial changes in TGFβ1 concentrations were characterized in 33 OS-bearing dogs before and after institution of effective palliative treatment comprised of ionizing radiation and IV zoledronate. The aver-age pretreatment plasma TGFβ1 concentration of 18.6 ± 8.7 ng/mL was subsequently decreased to 13.8 ± 8.8 ng/mL after effective focal malignant osteolysis management (Fig 6; P = .001).
Figure 6. Microscopic appearance (A) of spontaneous canine OS sample by hematoxylin and eosin staining (left panel) and confirmed expressions of TGFβRI (middle panel) and TGFβRII (right panel) in malignant osteoblasts identified by immunohistochemical staining. In 33 treatment-naïve OS-bearing dogs, (B) positive correlation between baseline plasma TGFβ1 concentrations and urine N-telopeptide excretions. Decreases in TGFβ1 concentrations (C) in serially collected plasma samples derived from 33 dogs treated with standardized palliative treatment inclusive of ionizing radiation treatment and IV zoledronate administration. Data expressed as mean ± SD. *P < .05.

Discussion
The biologic functions of TGFβ signaling actively participate in key cellular pathways, including apoptosis, cell cycle arrest, and immunity.[32-35] In addition to normal physiologic processes, TGFβ's canonical and noncanonical signaling pathways probably orchestrate various tumorigenic properties, including cancer cell proliferation, migration, survival, and angiogenesis.[1, 36] With respect to malignant bone pathology, TGFβ has been identified as a key driver in perpetuating the successful development and progression of skeletal metastases, particularly for breast carcinoma.[37-39] In the context of skeletal metastases, carcinoma cells successfully reaching the fertile bone microenvironment will subvert and dysregulate osteoclastic activities, thereby promoting excessive bone resorption, and consequently facilitate the release of TGFβ1 from the bone matrix.[40, 41] Serving as a potent mitogen for metastatic carcinoma cells, bone-derived TGFβ1 promotes carcinoma progression and accelerates additional osteoclastic bone resorption, with the establishment of a paracrine feedback loop termed the “vicious cycle”.[39]
Although definitively a driver of skeletal metastases biology, the involvement of TGFβ signaling for the establishment, maintenance, or progression of primary bone tumors, such as OS, remains incompletely investigated. Intuitively, given the role of TGFβ in reparative bone biology in which Smad-dependent and -independent signaling are responsible for normal osteoblast migration, proliferation, and survival,[14, 42-44] it would be rational to speculate that TGFβ signaling might also contribute to OS biology. Indeed, prior studies have investigated whether expression of different TGFβ ligand isoforms in tumor samples might influence OS biologic behavior in human patients,[45-47] as well as if specific TGFβ receptor gene polymorphisms might serve as risk factors for OS development.[48, 49] Extending upon studies that only characterized either ligand or receptor, more comprehensive investigations suggest cooperation between TGFβ receptors and cognate ligands in the form of an autocrine feedback loop with the capacity to promote OS cell growth or drug resistance.[50-52]
Complementing the results derived from studies of human and murine OS cells, the findings from the current investigation demonstrate that canine malignant osteoblasts similarly possess TGFβ receptor/ligand signaling machinery. All canine cells of osteoblast lineage, normal and malignant, expressed both TGFβRI and TGFβRII, suggesting that TGFβ signaling might not represent a malignant driver phenotype, but rather serve as a conserved pathway utilized by both reparative and malignant osteoblasts, alike. In addition, canine OS cells spontaneously secreted TGFβ1 at low ng/ml concentrations, a systemic concentration of cytokine that is capable of exerting active biologic effects in vivo.[53] Based upon the coexpression of both receptor and cognate ligand, it is plausible that canine OS cells have the potential to exploit either autocrine or paracrine TGFβ signaling as a mechanism favoring protumorigenesis.
Given the potential for the existence of an autocrine/paracrine signaling loop, in conjunction with the pivotal role that TGFβ plays in cell cycle regulation and epithelial-mesenchymal transition (EMT), the current study explored the effects on canine OS cell proliferation and migration after exposure to LY2109761, a highly selective and potent small molecule inhibitor of TGFβRI and TGFβRII. The ability of LY2109761 to effectively block canonical TGFβ-mediated signaling in canine OS cells was confirmed by the complete loss or substantial reduction in Smad 2 phosphorylation after stimulation with either 10% FBS alone or in conjunction with exogenous TGFβ1, respectively. With regard to OS cell proliferation, a dose-dependent antimitogenic effect of LY2109761 was demonstrated in both the HMPOS and Abrams OS cell lines. Although maximal decreases in colony formation and size were observed at the highest concentration of LY2109761 (5 μM) and correlated well with attenuating Smad 2 phosphorylation, the antiproliferative activity of LY2109761 was operative at concentrations as low as 0.3 μM, a finding consistent with the reported inhibition constant (Ki) of LY2109761 for TGFβRII.[54]
In addition to cell proliferation, TGFβ signaling blockade with LY2109761 decreased canine OS cell migration. The extent of impaired migratory ability appeared cell line dependent, with the motility of D17 and Abrams OS cell lines being markedly and minimally attenuated, respectively. Given that the highest concentration of LY2109761 (5 μM) used in this study was expected to completely block canonical and noncanonical TGFβ signaling, and yet the capacity of OS cells to migrate was not abolished completely, can be reconciled by alternate and redundant signaling pathways, such as c-Met,[55, 56] potentially exploited by canine OS for the preservation of cell motility.
Another pro-tumorigenic property investigated in this study was the influence of TGFβ signaling on the secretion of VEGF by canine OS cells. As a proangiogenic cytokine, TGFβ augments VEGF production by stabilizing HIF-1α indirectly by selectively inhibiting the transcription and translation of HIF-1α-associated prolyl hydroxylase (PHD), a negative regulator of HIF-1α stability.[57] Exposure to LY2109761 resulted in a dose-dependent decrease in secreted VEGF in all OS lines evaluated, with maximal VEGF reductions approaching 50%. Importantly, the postulated mechanism for the observed decreases in VEGF was supported by the documented decreases in HIF-1α, possibly through decreased stability, after exposure to LY2109761. Furthermore, the observed decreases in HIF-1α in close temporal relationship with LY2109761 exposure are not unexpected, given the rapid posttranslational regulation of HIF-1α by proteasome degradation, and consequent short HIF-1α half-life (<5 minutes).[58]
The potential translational relevance of TGFβ signaling in dogs diagnosed with OS initially was investigated by evaluation of TGFβRI and TGFβRII expression in 10 archived osteoblastic OS tissue samples. Consistent with the findings derived from immortalized canine OS cell lines, all 10 spontaneously arising OS samples demonstrated moderately strong and uniform positive staining for both receptors. Given that bone serves as the largest anatomic reservoir for TGFβ1 in the body, in conjunction with the focally osteolytic nature of OS, a correlative relationship between plasma TGFβ1 and urine N-telopeptide in 33 treatment-naïve OS-bearing dogs was explored and found to be significant. In the same 33 dogs, to indirectly support the notion that not only focal malignant osteolysis but also viable OS cells might contribute to circulating plasma TGFβ1 concentrations, serial plasma TGFβ1 concentrations were compared in each dog before and after the institution of effective palliative treatment comprised of standardized ionizing radiation and IV zoledronate. Not unexpectedly, in the majority of dogs (24/33), plasma TGFβ1 concentrations were substantially decreased after the institution of palliative treatment expected to decrease malignant osteolysis and induce OS cell apoptosis.
The collective findings of TGFβRI/II expression in OS samples and correlation between plasma TGFβ1 and focal osteolysis in OS-bearing dogs support the possibility that TGFβ signaling might contribute to OS progression, and hence blockade of TGFβ-mediated effects could be a rational therapeutic strategy. Although prolonged or complete TGFβ blockade would carry the risk for development of severe adverse effects, including lymphoproliferative disorders and exacerbation of orthopedic pathology such as osteoarthritis, several potential blocking strategies and dosing regimens appear to be viable for the management of fibrotic diseases and cancer.[59] Based upon a large body of preclinical efficacy studies, coupled with early promising results in Phase 1, 2, and 3 studies in which TGFβ blocking strategies are being evaluated for the management of diverse cancers in people, further investigations are clinically warranted to explore the suitability of inhibiting TGFβ signaling as an adjuvant treatment in dogs with OS.
Although the findings from this study provide incremental advances in the potential role of TGFβ signaling in OS, there are several limitations in the current investigation that should be recognized. First, no direct evidence, in vitro or in vivo, was provided to support the existence of a TGFβ-mediated autocrine/paracrine feedback loop in canine OS, and despite the demonstrated expression of cognate receptor/ligand pairs in OS cells, the biologic relevance of the identified cellular machinery cannot be ascertained. Second, although LY2109761 attenuated several pro-tumorigenic characteristics in vitro, the concentrations (5 μM) and exposure durations (24–48 hours) might not be biologically achievable or relevant in dogs with OS. However, preclinical murine models of skeletal metastases suggest that LY2109761 is tolerated and possesses favorable pharmacokinetics, as well as allowing for anticancer activities to be exerted within the bone microenvironment.[60, 61] Third, although LY2109761 decreased VEGF secretion in all canine OS cell lines tested, it cannot be concluded that this result is a direct consequence of decreased HIF-1α protein stability after TGFβ signaling blockade or simply an experimental design epiphenomenon. Fourth, the contributory origins of plasma TGFβ1 measured in OS-bearing dogs cannot be stated definitively. Nominal platelet aggregation, despite collection of blood in heparinized tubes, could have randomly influenced plasma TGFβ1 concentrations. Last, the decreases in plasma TGFβ1 after institution of ionizing radiation and IV zoledronate could not be strictly attributed to decreases in focal malignant osteolysis and OS cell viability, because whole skeletal antiresorptive effects of zoledronate would be anticipated to produce similar TGFβ1 reductive tracing.
In conclusion, findings from this investigation are novel from the perspective of canine OS biology, and corroborate existing studies conducted in human and murine OS cell lines. Future pharmacokinetic and tolerability studies in dogs with TGFβ blocking molecules, such as LY2109761, will be necessary to guide the formulation of appropriate dosing regimens. The generation of preliminary and interesting data in this report provides impetus to further evaluate the feasibility and suitability of TGFβ blocking strategies for the management of canine OS.
Acknowledgments
The authors thank the technicians and veterinary oncology residents of the University of Illinois Cancer Care Clinic for their contributions in this study.
Conflict of Interest Declaration: Timothy M. Fan serves as the Associate Editor for Oncology/Hematology in the Journal of Veterinary Internal Medicine.
-
1
-
2
Selleckchem, Houston, TX
-
3
BD Bioscience, San Jose, CA (product no. 610842)
-
4
Millipore, Billerica, MA (product no. 04-953)
-
5
Abcam, Cambridge, MA (product nos. ab31013, ab61213, ab8227, ab463)
-
6
Cell Signalling, Danvers, MA (product nos. 9271 and 9272)
-
7
Pierce, Rockford, IL
-
8
Protease Inhibitor Cocktail kit, Thermo Scientific, Waltham, MA
-
9
Amersham ECL kit, GE HealthCare Life Sciences, Piscataway, NJ
-
10
Image J Software, National Institutes of Health, Bethesda, MD
-
11
CellTiter 96® AQueous One Solution Cell Proliferation Assay, Promega, Madison, WI
-
12
Osteomark N-telopeptide urine ELISA, Alere Inc, Waltham, MA
References
-
1
Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet 2001;29:117–129.
-
2
Kubiczkova L, Sedlarikova L, Hajek R, et al. TGF-beta - an excellent servant but a bad master. J Transl Med 2012;10:183.
-
3
Lyons RM, Gentry LE, Purchio AF, et al. Mechanism of activation of latent recombinant transforming growth factor beta 1 by plasmin. J Cell Biol 1990;110:1361–1367.
-
4
Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev 2000;14:163–176.
-
5
Taipale J, Miyazono K, Heldin CH, et al. Latent transforming growth factor-beta 1 associates to fibroblast extracellular matrix via latent TGF-beta binding protein. J Cell Biol 1994;124:171–181.
-
6
Assoian RK, Komoriya A, Meyers CA, et al. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem 1983;258:7155–7160.
-
7
Bonewald LF, Mundy GR. Role of transforming growth factor-beta in bone remodeling. Clin Orthop Relat Res 1990;250:261–276.
-
8
Sigal LH. Basic science for the clinician 57: Transforming growth factor beta. J Clin Rheumatol 2012;18:268–272.
-
9
Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci 2005;118(Pt 16):3573–3584.
-
10
Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res 2009;19:128–139.
-
11
Centrella M, Horowitz MC, Wozney JM, et al. Transforming growth factor-beta gene family members and bone. Endocr Rev 1994;15:27–39.
-
12
Janssens K, ten Dijke P, Janssens S, et al. Transforming growth factor-beta1 to the bone. Endocr Rev 2005;26:743–774.
-
13
Oreffo RO, Mundy GR, Seyedin SM, et al. Activation of the bone-derived latent TGF beta complex by isolated osteoclasts. Biochem Biophys Res Commun 1989;158:817–823.
-
14
Bostrom MP, Asnis P. Transforming growth factor beta in fracture repair. Clin Orthop Relat Res 1998;355(Suppl):S124–S131.
-
15
Sarahrudi K, Thomas A, Mousavi M, et al. Elevated transforming growth factor-beta 1 (TGF-beta1) levels in human fracture healing. Injury 2011;42:833–837.
-
16
Alliston T, Choy L, Ducy P, et al. TGF-beta-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J 2001;20:2254–2272.
-
17
Iqbal J, Sun L, Zaidi M. Coupling bone degradation to formation. Nat Med 2009;15:729–731.
-
18
Maeda S, Hayashi M, Komiya S, et al. Endogenous TGF-beta signaling suppresses maturation of osteoblastic mesenchymal cells. EMBO J 2004;23:552–563.
-
19
Tang Y, Wu X, Lei W, et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med 2009;15:757–765.
-
20
Chun R, de Lorimier LP. Update on the biology and management of canine osteosarcoma. Vet Clin North Am Small Anim Pract 2003;33:491–516, vi.
-
21
Fan TM, de Lorimier LP, Garrett LD, et al. The bone biologic effects of zoledronate in healthy dogs and dogs with malignant osteolysis. J Vet Intern Med 2008;22:380–387.
-
22
Lacoste H, Fan TM, de Lorimier LP, et al. Urine N-telopeptide excretion in dogs with appendicular osteosarcoma. J Vet Intern Med 2006;20:335–341.Direct Link:
-
23
Schmit JM, Pondenis HC, Barger AM, et al. Cathepsin K expression and activity in canine osteosarcoma. J Vet Intern Med 2012;26:126–134.
-
24
Bacon NJ, Ehrhart NP, Dernell WS, et al. Use of alternating administration of carboplatin and doxorubicin in dogs with microscopic metastases after amputation for appendicular osteosarcoma: 50 cases (1999-2006). J Am Vet Med Assoc 2008;232:1504–1510.
-
25
Bailey D, Erb H, Williams L, et al. Carboplatin and doxorubicin combination chemotherapy for the treatment of appendicular osteosarcoma in the dog. J Vet Intern Med 2003;17:199–205.Direct Link:
-
26
Kent MS, Strom A, London CA, et al. Alternating carboplatin and doxorubicin as adjunctive chemotherapy to amputation or limb-sparing surgery in the treatment of appendicular osteosarcoma in dogs. J Vet Intern Med 2004;18:540–544.Direct Link:
-
27
Moore AS, Dernell WS, Ogilvie GK, et al. Doxorubicin and BAY 12-9566 for the treatment of osteosarcoma in dogs: A randomized, double-blind, placebo-controlled study. J Vet Intern Med 2007;21:783–790.Direct Link:
-
28
Gold LI. The role for transforming growth factor-beta (TGF-beta) in human cancer. Crit Rev Oncog 1999;10:303–360.
-
29
Jakowlew SB. Transforming growth factor-beta in cancer and metastasis. Cancer Metastasis Rev 2006;25:435–457.
-
30
Kaminska B, Wesolowska A, Danilkiewicz M. TGF beta signalling and its role in tumour pathogenesis. Acta Biochim Pol 2005;52:329–337.
-
31
Xu CC, Wu LM, Sun W, et al. Effects of TGF-beta signaling blockade on human A549 lung adenocarcinoma cell lines. Mol Med Rep 2011;4:1007–1015.
-
32
Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol 2002;2:46–53.
-
33
Sporn MB, Roberts AB, Wakefield LM, et al. Transforming growth factor-beta: Biological function and chemical structure. Science 1986;233:532–534.
-
34
Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer 2003;3:807–821.
-
35
Schuster N, Krieglstein K. Mechanisms of TGF-beta-mediated apoptosis. Cell Tissue Res 2002;307:1–14.
-
36
Elliott RL, Blobe GC. Role of transforming growth factor Beta in human cancer. J Clin Oncol 2005;23:2078–2093.
-
37
Buijs JT, Stayrook KR, Guise TA. The role of TGF-beta in bone metastasis: Novel therapeutic perspectives. Bonekey Rep 2012;1:96.
- 38
-
39
Guise TA, Chirgwin JM. Transforming growth factor-beta in osteolytic breast cancer bone metastases. Clin Orthop Relat Res 2003;415(Suppl):S32–S38.
-
40
Futakuchi M, Nannuru KC, Varney ML, et al. Transforming growth factor-beta signaling at the tumor-bone interface promotes mammary tumor growth and osteoclast activation. Cancer Sci 2009;100:71–81.
-
41
Sato S, Futakuchi M, Ogawa K, et al. Transforming growth factor beta derived from bone matrix promotes cell proliferation of prostate cancer and osteoclast activation-associated osteolysis in the bone microenvironment. Cancer Sci 2008;99:316–323.
-
42
Linkhart TA, Mohan S, Baylink DJ. Growth factors for bone growth and repair: IGF, TGF beta and BMP. Bone 1996;19(1 Suppl):1S–12S.
-
43
Karsdal MA, Larsen L, Engsig MT, et al. Matrix metalloproteinase-dependent activation of latent transforming growth factor-beta controls the conversion of osteoblasts into osteocytes by blocking osteoblast apoptosis. J Biol Chem 2002;277:44061–44067.
-
44
Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res 2002;17:513–520.
-
45
Kloen P, Gebhardt MC, Perez-Atayde A, et al. Expression of transforming growth factor-beta (TGF-beta) isoforms in osteosarcomas: TGF-beta3 is related to disease progression. Cancer 1997;80:2230–2239.
-
46
Yang RS, Wu CT, Lin KH, et al. Relation between histological intensity of transforming growth factor-beta isoforms in human osteosarcoma and the rate of lung metastasis. Tohoku J Exp Med 1998;184:133–142.
-
47
Franchi A, Arganini L, Baroni G, et al. Expression of transforming growth factor beta isoforms in osteosarcoma variants: Association of TGF beta 1 with high-grade osteosarcomas. J Pathol 1998;185:284–289.Direct Link:
-
48
Hu YS, Pan Y, Li WH, et al. Association between TGFBR1*6A and osteosarcoma: A Chinese case-control study. BMC Cancer 2010;10:169.
-
49
Hu YS, Pan Y, Li WH, et al. Int7G24A variant of transforming growth factor-beta receptor 1 is associated with osteosarcoma susceptibility in a Chinese population. Med Oncol 2011;28:622–625.
-
50
Kloen P, Jennings CL, Gebhardt MC, et al. Expression of transforming growth factor-beta (TGF-beta) receptors, TGF-beta 1 and TGF-beta 2 production and autocrine growth control in osteosarcoma cells. Int J Cancer 1994;58:440–445.Direct Link:
- 51
-
52
Liu Y, Zheng QX, Du JY, et al. Effects of TGF beta1 autocrine blockage on osteosarcoma cells. Chin Med Sci J 2004;19:155–156.
-
53
Wakefield LM, Letterio JJ, Chen T, et al. Transforming growth factor-beta1 circulates in normal human plasma and is unchanged in advanced metastatic breast cancer. Clin Cancer Res 1995;1:129–136.
-
54
Li HY, McMillen WT, Heap CR, et al. Optimization of a dihydropyrrolopyrazole series of transforming growth factor-beta type I receptor kinase domain inhibitors: Discovery of an orally bioavailable transforming growth factor-beta receptor type I inhibitor as antitumor agent. J Med Chem 2008;51:2302–2306.
-
55
Liao AT, McCleese J, Kamerling S, et al. A novel small molecule Met inhibitor, PF2362376, exhibits biological activity against osteosarcoma. Vet Comp Oncol 2007;5:177–196.
-
56
Liao AT, McMahon M, London CA. Identification of a novel germline MET mutation in dogs. Anim Genet 2006;37:248–252.
-
57
McMahon S, Charbonneau M, Grandmont S, et al. Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J Biol Chem 2006;281:24171–24181.
-
58
Jewell UR, Kvietikova I, Scheid A, et al. Induction of HIF-1alpha in response to hypoxia is instantaneous. FASEB J 2001;15:1312–1314.
-
59
Calone I, Souchelnytskyi S. Inhibition of TGFbeta signaling and its implications in anticancer treatments. Exp Oncol 2012;34:9–16.
-
60
Wan X, Li ZG, Yingling JM, et al. Effect of transforming growth factor beta (TGF-beta) receptor I kinase inhibitor on prostate cancer bone growth. Bone 2012;50:695–703.
-
61
Ganapathy V, Ge R, Grazioli A, et al. Targeting the Transforming Growth Factor-beta pathway inhibits human basal-like breast cancer metastasis. Mol Cancer 2010;9:122.
Figure S1. Visually evident (A) and quantifiable (B) decrease in formed colony diameter exerted by LY2109761 in the HMPOS cell line after 10 days of undisturbed growth. Similar quantitative (C) attenuations in size of colonies formed with LY2109761 exposure in the Abrams cell line after 7 days of undisturbed growth. Data expressed as mean ± SD. *P < .05 and **P < .01 in comparison with DMSO control.

Share this article / Teilen Sie diesen Artikel
Diese Webseite wurde mit Jimdo erstellt! Jetzt kostenlos registrieren auf https://de.jimdo.com








